Abstract
Purpose
To assess the clinical utility of a recently developed highly sensitive cardiac troponin T (hs-cTnT) assay for providing prognostic information on patients with sepsis.
Methods
cTnT levels were measured by the novel hs-cTnT assay at two time points (inclusion and 72 h thereafter) in a subgroup of patients from the FINNSEPSIS study and associations with clinical outcomes were examined. Results for the hs-cTnT assay were compared to those of the established fourth-generation cTnT assay.
Results
cTnT measured by the fourth-generation and hs-cTnT assay was detectable in 124 (60%) and 207 (100%) patients, respectively, on inclusion in this study. hs-cTnT levels on inclusion correlated with several indices of risk in sepsis, including the simplified acute physiology score (SAPS) II and sequential organ failure assessment (SOFA) scores. The level of hs-cTnT on inclusion was higher in hospital non-survivors (n = 47) than survivors (n = 160) (median 0.054 [Q1–3, 0.022–0.227] versus 0.035 [0.015–0.111] μg/L, P = 0.047), but hs-cTnT level was not an independent predictor of in-hospital mortality. hs-cTnT levels on inclusion were also higher in patients with septic shock during the hospitalization (0.044 [0.024–0.171] versus 0.033 [0.012–0.103] μg/L, P = 0.03), while this was not the case for the fourth-generation cTnT assay or NT-proBNP levels.
Conclusions
Circulating hs-cTnT is present in patients with severe sepsis and septic shock, associates with disease severity and survival, but does not add to SAPS II score for prediction of mortality. hs-cTnT measurement could still have a role in sepsis as an early marker of shock.
Similar content being viewed by others
Introduction
Severe sepsis and septic shock are commonly complicated by myocardial dysfunction, even in patients without pre-existing coronary artery disease [1, 2], and cardiac failure may contribute to poor outcome in these patients [1, 2]. Early recognition of myocardial injury and ventricular dysfunction in severe sepsis and septic shock may improve short-term prognosis, but is difficult to diagnose [3].
Cardiac troponins T (cTnT) and I (cTnI) are components of the myofibrillar contractile apparatus of cardiomyocytes. Measurement of troponins is routinely used for diagnosis of myocardial necrosis in patients with suspected acute coronary syndromes [4, 5], and to identify patients benefiting from early medical and coronary interventions [6, 7]. Recently, several studies have also reported detectable cTnT or cTnI levels in patients with sepsis, but their predictive value has not been firmly established [8–16]. Explanations for the diverging results regarding utility of troponin testing in sepsis may be inadequate sample size and use of troponin assays with limited sensitivity and precision in the low concentration range [8–16]. However, a new and highly sensitive assay for cTnT (hs-cTnT) measurement now allows detection of concentrations tenfold lower than with previous assays [17]. The clinical benefit of using this novel hs-cTnT assay has been demonstrated in patients with stable coronary artery disease and preserved left ventricular function, as the hs-cTnT assay detected measurable levels of troponin T in 97.7% of these patients, and improved risk stratification beyond standard patient evaluation [18]. In contrast, only 1.3% of these patients would have had measurable levels of cTnT with the conventional fourth-generation assay.
Accordingly, we hypothesized that hs-cTnT would be detectable in a high proportion of patients with sepsis or septic shock, be associated with conventional risk markers in sepsis, and provide superior prognostic information compared to a conventional fourth-generation cTnT assay.
Materials and methods
Study design
The study is a substudy of FINNSEPSIS, a prospective observational cohort study of the incidence and prognosis of sepsis in 24 intensive care units (ICUs) in Finland [19, 20]. In brief, all patients aged 18 years or older admitted to the ICUs of participating centres were screened daily for the American College of Chest Physicians/Society of Critical Care Medicine criteria of severe sepsis or septic shock. All patients fulfilling the criteria, i.e. known or suspected infection, with two or more criteria for systemic inflammatory response syndrome, and at least one new, sepsis-induced organ failure, were included in the FINNSEPSIS cohort. Previous diagnosis of cardiovascular disease (defined as either a history of heart failure or coronary artery disease requiring medication or former disease-associated procedure), hypertension, or diabetes mellitus was collected from patient chart records. Peak creatinine and lactate levels, the lowest platelet count, and maximal dose of vasoactive drugs infused during the first 24 h after inclusion were also recorded. After the first 24 h, the simplified acute physiology score (SAPS) II was recorded [21]. The sequential organ failure assessment (SOFA) was subsequently scored daily [22]. All data were collected and transferred via internet to the Finnish intensive care quality consortium (Intensium, Kuopio, Finland). In-hospital mortality was recorded and classified as the primary endpoint. Patients were screened daily for septic shock during the ICU stay, and shock was considered a secondary endpoint. The FINNSEPSIS study and all substudies have been approved by the Regional Ethics Committees at each participating centre, and the study was performed according to the Declaration of Helsinki.
Biochemical analyses
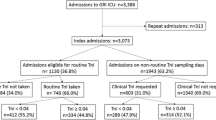
For this biomarker substudy, patients were included if written informed consent for blood sampling could be obtained from the patient or a relative, and if laboratory samples were available from both inclusion in the study and 72 h thereafter (Fig. 1). Blood sampling was performed from indwelling arterial catheters or by venipuncture [23, 24]. The samples were centrifuged at room temperature and the serum component was aspirated and frozen at −20°C at individual centres. Within 3 months of collection, plasma samples were shipped on dry ice to the Helsinki University Hospital for storage at −80°C for 4 years pending analysis at Akershus University Hospital.
hs-cTnT and cTnT levels were determined on an autoanalyzer (Cobas e 411) using only commercial assays (Roche Diagnostics, Penzberg, Germany). The hs-cTnT assay utilizes the same antibodies as the conventional fourth-generation assay [17]. The lower detection level of the hs-cTnT assay is 0.003 μg/L, and the 99th percentile value with less than 10% coefficient of variance (CV) in healthy subjects is 0.014 μg/L. The corresponding detection limit for the fourth-generation assay is 0.01 μg/L, and the 99th percentile value with less than 10% CV is 0.03 μg/L. The laboratory personnel were blinded to patients clinical information.
Estimated glomerulal filtration rate (eGFR) was calculated by the MDRD formula [25], N-terminal pro-B-type natriuretic peptide (NT-proBNP) was analysed as previously described [24], and body mass index (BMI) was calculated as weight (kg)/height (m)2.
Statistical analyses
Data are presented as median (quartile (Q) 1–3) or absolute values and percentages. We assessed differences between groups by the Mann–Whitney U test for continuous variables as most were not normally distributed, while categorical data were compared by the Fisher exact test. Associations between variables were assessed by Spearman rank correlation. Variables associated with hs-TnT levels (logarithmic transformation) on inclusion were evaluated by multivariate linear regression analysis with age, gender, previous cardiovascular disease, hypertension, diabetes, BMI, NT-proBNP, eGFR, and peak lactate levels and lowest platelet count within the first 24 h from inclusion in the model (logarithmic transformation for the last four variables). Odds ratios (OR) with 95% confidence interval (CI) for in-hospital mortality were calculated by logistic regression analysis. Because of skewed distributions, troponin T measurements were entered after logarithmic transformation. Predictive factors that were significant in univariate analysis were included in a multivariate model using a forward selection procedure. Receiver operating characteristics (ROC) curve analysis was used for identification of optimal cut-off points and to examine discrimination, and areas under the curve (AUCs with 95% CI) were compared [26]. P values less than 0.05 were considered significant for all analyses. Statistical analyses were performed with SPSS for Windows version 14.0 (SPSS, Chicago, IL) with the exception of the comparison of ROC AUCs, which was performed with MedCalc for Windows, version 9.5.1.0 (MedCalc Software, Mariakerke, Belgium) and the statistical programming language R (R Development Core Team, 2008).
Results
Patient characteristics
We included all patients who gave written informed consent and for whom blood samples were available at both time points examined: (1) inclusion in this study and (2) 72 h thereafter. This accounted for 207 out of the 470 patients (44%) from the FINNSEPSIS cohort (Fig. 1). Median duration from diagnosis of sepsis to blood sampling was 9 h (Q1–3, 3–17 h). Of these 207 patients, 47 (23%) died in the hospital. Characteristics of the patients according to in-hospital mortality are summarized in Table 1.
Cardiac-specific troponins
cTnT measured by the fourth-generation assay was not detectable in 83 (40%) of the patients on inclusion in the study, while 87 patients (43%) had undetectable levels 72 h after inclusion. In contrast, cTnT measured by the highly sensitive assay was detectable in all patients at both time points. On inclusion, hs-cTnT levels ranged from 0.004 to 13.577 μg/L (median 0.039 μg/L [Q1–3, 0.017–0.135 μg/L]), whereas after 72 h concentrations ranged from 0.003 to 6.580 μg/L (median 0.037 μg/L [Q1–3, 0.016–0.109 μg/L]). Median cTnT levels measured by the fourth-generation assay were 0.02 μg/L at both time points (Q1–3, 0.00–0.11 and 0.00–0.09 μg/L, respectively). On inclusion, 166 patients (80%) had cTnT levels over the normal 99th percentile value according to the hs-cTnT assay (>0.014 μg/L) versus 86 patients (42%) according to the fourth-generation cTnT assay (>0.03 μg/L) (Supplementary Figure). Similarly, hs-cTnT levels were elevated in 161 patients (of 204, 79%) 72 h after inclusion compared to 14 patients (7%) according to the fourth-generation assay (Supplementary Figure). Characteristics of the patients according to hs-cTnT elevation on inclusion in this study are shown in the online supplementary material (Supplementary Table 1).
A correlation was observed between hs-cTnT levels on inclusion and after 72 h (n = 204; r = 0.82, P < 0.001). hs-cTnT levels on inclusion were also correlated with severity of disease (SAPS II score, r = 0.27, P < 0.001), multiple organ dysfunction (SOFA score, r = 0.30, P < 0.001), creatinine level (r = 0.32, P < 0.001), age (r = 0.22, P = 0.002), and peak 24-h dose of dobutamine (r = 0.15, P = 0.03) and noradrenalin (r = 0.19, P = 0.007). Elevated NT-proBNP levels on inclusion (P < 0.001), previous cardiovascular disease (P = 0.005), and increased BMI levels (P = 0.015) were associated with high levels of hs-cTnT on study inclusion, whereas there was no significant association with patient age, gender, hypertension, diabetes, platelet count, eGFR or lactate levels (Supplementary Table 2). The model of NT-proBNP levels, previous cardiovascular disease, and BMI explained 42% of the variance in hs-cTnT levels (r 2 = 0.42).
Troponin T levels and in-hospital mortality
The median hs-cTnT level on inclusion in this study was higher in hospital non-survivors than in survivors: 0.054 (Q1–3, 0.022–0.227) versus 0.035 (0.015–0.111) μg/L, P = 0.047 (Fig. 2), while the fourth-generation assay did not differentiate between hospital non-survivors and survivors: 0.04 (0.00–0.21) versus 0.02 (0.00–0.09) μg/L, P = 0.14. cTnT levels after 72 h were not different for patients surviving to hospital discharge and non-survivors, either measured by the highly sensitive assay: 0.054 (0.019–0.224) versus 0.035 (0.015–0.093) μg/L, P = 0.054, or by the fourth-generation assay: 0.03 (0.00–0.20) versus 0.01 (0.00–0.08) μg/L, P = 0.11. Change in cTnT levels from inclusion in this study to 72 h was not associated with in-hospital mortality: neither according to the high sensitivity assay: non-survivors: 0.001 (−0.033 to 0.015) versus survivors: −0.004 (−0.028 to 0.003) μg/L (P = 0.63), nor the fourth-generation cTnT assay: non-survivors: 0.00 (−0.03 to 0.02) versus survivors: 0.00 (−0.02 to 0.00) μg/L (P = 0.52). In the subgroup of patients with moderate severity of disease (inframedian SAPS II score <47 points, n = 97), hs-cTnT levels were not different in hospital non-survivors (n = 15) compared to survivors (n = 82): 0.042 (0.018–0.242) versus 0.030 (0.012–0.098) μg/L (P = 0.17). In patients with undetectable cTnT levels with the fourth-generation assay on inclusion (n = 83), no significant differences in hs-cTnT levels in non-survivors (n = 16) versus survivors (n = 67) were found: 0.017 (0.011–0.023) versus 0.014 (0.008–0.021) (P = 0.11).
hs-cTnT levels on inclusion in this study and 72 h thereafter in patients surviving to hospital discharge and in non-survivors. The horizontal line within the box represents the median level, the boundaries of the box quartiles 1–3, and the whiskers range (maximum value restricted to 1.5× interquartile range from the median). # P < 0.05
By univariate logistic regression, inclusion hs-cTnT levels (OR 1.29 [95% CI 1.03–1.60]) and levels after 72 h (OR 1.28 [1.04–1.58]) were both associated with in-hospital mortality (Table 2). AUCs for hs-cTnT on study inclusion and after 72 h were comparable: AUC 0.588 (95% CI 0.517–0.656) vs. 0.593 (0.522–0.661). Inclusion or 72 h cTnT measured by the fourth-generation assay was not associated with in-hospital mortality: ORs 1.12 [95% CI 0.98–1.27] and 1.12 [0.99–1.28], respectively).
After adjustment for age, previous cardiovascular disease, eGFR, peak lactate levels, SAPS II and SOFA scores in multivariate analysis, neither hs-cTnT nor cTnT measured by the fourth-generation assay was associated with in-hospital mortality (P values 0.26 and 0.65, respectively; Table 2). Furthermore, the addition of cTnT measurement to SAPS II score did not improve discrimination between hospital survivors and non-survivors: AUC = 0.678 for SAPS II score alone, AUC = 0.685 for SAPS II plus fourth-generation cTnT assay, and AUC = 0.684 for SAPS II plus hs-cTnT, both P = 0.45 vs. SAPS II alone (Fig. 3).
Troponin T levels and septic shock
hs-cTnT levels on inclusion in this study were higher in patients with septic shock during the hospitalization compared to those without shock: 0.044 (Q1–3, 0.024–0.171) versus 0.033 (0.012–0.103) μg/L (P = 0.03; Fig. 4). In contrast, cTnT levels measured by the fourth-generation assay were comparable: 0.02 (0.00–0.15) versus 0.01 (0.00–0.09) μg/L (P = 0.10), as were NT-proBNP levels on inclusion: 4,809 (2,064–9,782) versus 3,631 (764–12,351) pg/mL (P = 0.17; Fig. 4). The optimal cut-off for hs-cTnT to predict septic shock during the hospitalization (86% sensitivity, 33% specificity) was 0.014 μg/L. Cut-off points for the fourth-generation cTnT assay and NT-proBNP on inclusion were 0.01 μg/L (58% sensitivity, 51% specificity) and 785 pg/mL (90% sensitivity, 26% specificity), respectively. In a multivariate model adjusting for other cardiac biomarkers and information that were available on inclusion in this study (Supplementary Table 3), hs-cTnT greater than 0.014 μg/L was independently associated with shock during the hospitalization (OR 2.45 [95% CI 1.09–5.53], P = 0.03).
hs-cTnT and NT-proBNP levels on inclusion in this study in patients with circulatory failure and non-failure during the index hospitalization. The horizontal line within the box represents the median level, the boundaries of the box quartiles 1–3, and the whiskers range (maximum value restricted to 1.5× interquartile range from the median). # P < 0.05
Discussion
The novel findings of the current study of a large cohort of patients with severe sepsis are that (1) detectable levels of cTnT are present in all patients according to the new highly sensitive assay, whereas detectable levels were observed in 60% of patients by using a conventional assay; (2) hs-cTnT correlated with disease severity in severe sepsis and septic shock; and (3) although hs-cTnT levels were significantly higher in hospital non-survivors than in survivors, the discriminatory ability was only modest; and (4) hs-cTnT levels may have greatest potential in assessing risk for septic shock.
Several studies have reported an association between troponin levels, measured by conventional assays and short-term outcome in severe sepsis or septic shock [8, 9, 12, 13, 15, 27], whereas others have shown no relationship [10, 16]. Recently, a study of a cohort of 159 patients with bacteraemia reported that cTnI measured with a conventional assay is a univariate, but not an independent predictor of outcome [14]. In another study with 218 paediatric septic or septic shock patients the same pattern was observed [28]. The current study, which to our knowledge is the first to evaluate the novel highly sensitive assay and includes the largest cohort of adult septic patients with troponin measurements, confirms and extends the data regarding short-term risk stratification in severe sepsis. Moreover, the study results suggest that hs-cTnT measurement, despite reflecting cardiomyocyte injury, does not reliably identify non-surviving patients. Thus, although we used a highly sensitive cTnT assay, closely associated with traditional indices of risk, and identified low-level myocardial troponin release in a greater proportion of patients than the traditional fourth-generation troponin assay, this did not translate into significantly improved prediction of in-hospital mortality. This was also the case in subgroups of patients with intermediate risk and in the patients that had undetectable cTnT levels as measured by the fourth-generation assay. The lack of information regarding mortality could be attributable to myocardial dysfunction not being the principal mode of death in patients with severe sepsis, thus also diminishing the merit of hs-cTnT as a marker of mortality in sepsis. In contrast, hs-cTnT may still be of use in patients with severe sepsis for detection of shock. Interestingly, we found that the previously recognized hs-cTnT 99th percentile level of 0.014 μg/L in the general population was superior to other cardiac biomarkers and clinical variables for prediction of shock. Thus, a cTnT level less than 0.014 μg/L in the early stage of severe sepsis may be useful to identify patients with low risk of shock, which is in line with a strategy previously also proposed for NT-proBNP [29]. Still, this result needs to be confirmed in future studies, and especially in studies that also compare hs-cTnT measurement to echocardiographic findings.
As the cardiac-specific troponins differ from skeletal muscle-derived troponin isoforms, detection of cTnI and cTnT in the circulation has generally been considered a sensitive and specific marker of cardiomyocyte necrosis, and these proteins are today routinely used in the clinical context of acute coronary syndromes [4, 5]. Although a low-grade elevation of circulating troponin T levels can also be found in healthy individuals after strenuous physical exercise [30], the association between hs-cTnT levels and septic shock in our patients points to a role for hs-cTnT in sepsis as an indicator of myocardial function and circulatory status. This is also supported by a close association between hs-cTnT and NT-proBNP levels, and the higher hs-cTnT levels found in patients with established cardiovascular disease. Interestingly, elevated troponin levels have also been detected in other cardiac and non-cardiac conditions, including acute and chronic heart failure [31, 32], renal failure [33], chronic obstructive pulmonary disease [34, 35], pulmonary embolism [36], and sepsis [8–16, 27, 28, 37]. The mechanism for troponin elevation in these conditions may not be an acute coronary artery occlusion, but rather low-grade cardiomyocyte injury due to an unfavourable myocardial milieu [1, 2, 37]. Still, earlier studies of patients with sepsis have reported an association between troponin elevation and left ventricular dysfunction, as assessed by echocardiography and pulmonary artery catheterization [8–10, 12]. Potential contributing factors to troponin elevation in this setting are reduced myocardial blood flow secondary to arterial hypotension, microvascular thrombosis with subsequent myocardial injury and augmented myocardial apoptosis secondary to pro-inflammatory cytokines, including tumour necrosis factor-α, interleukin-1β, and interleukin-6 [1, 2]. Moreover, troponin release in sepsis does not necessarily indicate cardiomyocyte necrosis, but could also be a result of increased cell permeability and the release of troponin degradation products through the cell membrane in non-necrotic cardiomyocytes [38]. The value of troponin measurement for assessing myocardial function and cardiovascular status is supported by our study, but it should be recognized that diverging results have been reported for the cytokine-mediated troponin release [39] and the alleged impaired coronary blood flow in patients with septic shock [40].
Our study has some limitations. First, because of the exclusion of early deaths patients with consent for blood samples at both time points had slightly lower in-hospital mortality compared to all participants in the FINNSEPSIS study. Second, we were not able to include any objective measures of cardiac function, such as echocardiography. Finally, no assessment of coronary anatomy was performed, thus precluding assessment of the contribution of coronary stenosis to troponin elevation. Furthermore, these data do not translate to any conclusions for troponin I, as the mechanism for release and frequency of elevation may be different. The timing of blood sampling may also have affected the results for troponin measurement, although our daily screening strategy ensured that a high proportion of the samples were obtained early after start of sepsis.
In conclusion, we found that the novel highly sensitive assay detects circulating troponin T in all patients with severe sepsis, and that hs-cTnT levels are correlated with indices of poor prognosis in sepsis, but does not add to SAPS II score for prediction of in-hospital mortality. In contrast, hs-cTnT levels in the early phase of sepsis seem to provide interesting information regarding shock, and the major use of hs-cTnT measurement in sepsis could possibly be to stratify patients regarding risk of shock. Still, this needs to be verified in additional studies, and also compared to objective measures of cardiac function, as assessed by echocardiography or other imaging modalities.
References
Maeder M, Fehr T, Rickli H, Ammann P (2006) Sepsis-associated myocardial dysfunction: diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest 129:1349–1366
Fernandes CJ Jr, Akamine N, Knobel E (2008) Myocardial depression in sepsis. Shock 30 Suppl 1:S14–S17
Arlati S, Brenna S, Prencipe L, Marocchi A, Casella GP, Lanzani M, Gandini C (2000) Myocardial necrosis in ICU patients with acute non-cardiac disease: a prospective study. Intensive Care Med 26:31–37
Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH, National Academy of Clinical Biochemistry (2007) National Academy of Clinical Biochemistry laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 115:e356–e375
Gupta S, de Lemos JA (2007) Use and misuse of cardiac troponins in clinical practice. Prog Cardiovasc Dis 50:151–165
Hamm CW, Heeschen C, Goldmann B, Vahanian A, Adgey J, Miguel CM, Rutsch W, Berger J, Kootstra J, Simoons ML (1999) Benefit of abciximab in patients with refractory unstable angina in relation to serum troponin T levels. c7E3 Fab antiplatelet therapy in unstable refractory angina (CAPTURE) study investigators. N Engl J Med 340:1623–1629
Heeschen C, Hamm CW, Goldmann B, Deu A, Langenbrink L, White HD (1999) Troponin concentrations for stratification of patients with acute coronary syndromes in relation to therapeutic efficacy of tirofiban. PRISM study investigators. Platelet receptor inhibition in ischemic syndrome management. Lancet 354:1757–1762
Fernandes CJ Jr, Akamine N, Knobel E (1999) Cardiac troponin: a new serum marker of myocardial injury in sepsis. Intensive Care Med 25:1165–1168
Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR (2004) Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 95:13–17
Scott EC, Ho HC, Yu M, Chapital AD, Koss W, Takanishi DM Jr (2008) Pre-existing cardiac disease, troponin I elevation and mortality in patients with severe sepsis and septic shock. Anaesth Intensive Care 36:51–59
Spies C, Haude V, Fitzner R, Schroder K, Overbeck M, Runkel N et al (1998) Serum cardiac troponin T as a prognostic marker in early sepsis. Chest 113:1055–1063
ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK (2000) Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 46:650–657
Yucel T, Memis D, Karamanlioglu B, Sut N, Yuksel M (2008) The prognostic value of atrial and brain natriuretic peptides, troponin I and C-reactive protein in patients with sepsis. Exp Clin Cardiol 13:183–188
Kalla C, Raveh D, Algur N, Rudensky B, Yinnon AM, Balkin J (2008) Incidence and significance of a positive troponin test in bacteremic patients without acute coronary syndrome. Am J Med 121:909–915
Choon-ngarm T, Partpisanu P (2008) Serum cardiac troponin-T as a prognostic marker in septic shock. J Med Assoc Thai 91:1818–1821
Brivet FG, Jacobs FM, Colin P, Prat D, Grigoriu B (2006) Cardiac troponin level is not an independent predictor of mortality in septic patients requiring medical intensive care unit admission. Crit Care 10:404
Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA (2010) Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 56:642–650
Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E, Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) Trial Investigators (2009) A sensitive cardiac troponin T assay in stable coronary artery disease. New Engl J Med 361:2538–2547
Karlsson S, Varpula M, Ruokonen E, Pettilä V, Parviainen I, Ala-Kokko TI, Kolho E, Rintala EM (2007) Incidence, treatment, and outcome of severe sepsis in ICU-treated adults in Finland: the Finnsepsis study. Intensive Care Med 33:435–443
Varpula M, Karlsson S, Parviainen I, Ruokonen E, Pettilä V (2007) Community-acquired septic shock: early management and outcome in a nationwide study in Finland. Acta Anaesthesiol Scand 51:1320–1326
Jr LeGall, Lemeshow S, Saulnier F (1993) A new simplified acute physiology score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Bendel S, Karlsson S, Pettilä V, Loisa P, Varpula M, Ruokonen E (2008) Free cortisol in sepsis and septic shock. Anesth Analg 106:1813–1819
Varpula M, Pulkki K, Karlsson S, Ruokonen E, Pettilä V (2007) Predictive value of N-terminal pro-brain natriuretic peptide in severe sepsis and septic shock. Crit Care Med 35:1277–1283
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130:461–470
Hanley JA, McNeil BJ (1983) A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148:839–843
John J, Awab A, Norman D, Dernaika T, Kinasewitz GT (2007) Activated protein C improves survival in severe sepsis patients with elevated troponin. Intensive Care Med 33:2122–2128
Oliveira NS, Silva VR, Castelo JS, Elias-Neto J, Pereira FE, Carvalho WB (2008) Serum level of cardiac troponin I in pediatric patients with sepsis or septic shock. Pediatr Crit Care Med 9:414–417
Januzzi JL, Morss A, Tung R, Pino R, Fifer MA, Thompson BT, Lee-Lewandrowski E (2006) Natriuretic peptide testing for the evaluation of critical ill patients with shock in the intensive care unit: a prospective cohort study. Crit Care 10:134
Mingels A, Jacobs L, Michielsen E, Swaanenburg J, Wodzig W, van Dieijen-Visser M (2009) Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem 55:101–108
Sato Y, Yamada T, Taniguchi R, Nagai K, Makiyama T, Okada H, Kataoka K, Ito H, Matsumori A, Sasayama S, Takatsu Y (2001) Persistently increased serum concentrations of cardiac troponin T in patients with idiopathic dilated cardiomyopathy are predictive of adverse outcomes. Circulation 103:369–374
Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, Angelici L, Barlera S, Parrinello G, Maggioni AP, Tognoni G, Cohn JN, Val-HeFT Investigators (2007) Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 116:1242–1249
Apple FS, Murakami MM, Pearce LA, Herzog CA (2002) Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 106:2941–2945
Brekke PH, Omland T, Holmedal SH, Smith P, Søyseth V (2008) Troponin T elevation and long-term mortality after chronic obstructive pulmonary disease exacerbation. Eur Respir J 31:563–570
Brekke PH, Omland T, Holmedal SH, Smith P, Søyseth V (2009) Determinants of cardiac troponin T elevation in COPD exacerbation—a cross-sectional study. BMC Pulm Med 9:35
Konstantinides S, Geibel A, Olschewski M, Kasper W, Hruska N, Jackle S, Binder L (2002) Importance of cardiac troponins I and T in risk stratification of patients with acute pulmonary embolism. Circulation 106:1263–1268
Ammann P, Maggiorini M, Bertel O, Haenseler E, Joller-Jemelka HI, Oechslin E, Minder EI, Rickli H, Fehr T (2003) Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol 41:2004–2009
Wu AHB (2001) Increased troponin in patients with sepsis and septic shock: myocardial necrosis or reversible myocardial depression? Intensive Care Med 27:959–961
van Bockel EA, Tulleken JE, Muller Kobold AC, Ligtenberg JJ, van der Werf TS, Spanjersberg R, Zijlstra JG (2003) Cardiac troponin I release and cytokine response during experimental human endotoxaemia. Intensive Care Med 29:1598–1600
Cunnion RE, Schaer GL, Parker MM, Natanson C, Parrillo JE (1986) The coronary circulation in human septic shock. Circulation 73:637–644
Acknowledgments
We would like to thank all participating centres in the FINNSEPSIS biomarker substudy for patient recruitment and blood sampling. We thank Siri Mortensen and Linda Ellvin at the Center of Laboratory Medicine, Akershus University Hospital for skilful technical work. The contribution by Ståle Nygård, PhD to the statistics regarding AUCs is also appreciated. This study was supported by the University of Oslo. The sponsor had no role in any of the following: design and conduct of the study, collection, management, analysis and interpretation of the data, or preparation, review and approval of the manuscript.
Conflict of interest
TO has received lecture fees and grant support from Roche Diagnostics. No other potential conflict of interest relating to this article is reported.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
On behalf of the FINNSEPSIS Study Group. A complete list of the investigators who participated in the study is presented in “Appendix 1”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix 1
Appendix 1
A complete list of the investigators who participated in the study is presented in Table 3.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Røsjø, H., Varpula, M., Hagve, TA. et al. Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med 37, 77–85 (2011). https://doi.org/10.1007/s00134-010-2051-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-2051-x