Abstract
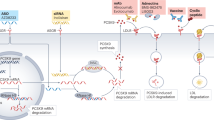
The burden of atherothrombotic cardiovascular disease remains high despite currently available optimum medical therapy. To address this substantial residual risk, the development of novel therapies that attempt to harness the atheroprotective functions of HDL is a major goal. These functions include the critical role of HDL in reverse cholesterol transport, and its anti-inflammatory, antithrombotic, and antioxidant activities. Discoveries in the past decade have shed light on the complex metabolic and antiatherosclerotic pathways of HDL. These insights have fueled the development of HDL-targeted drugs, which can be classified among four different therapeutic approaches: directly augmenting apolipoprotein A-I (apo A-I) levels, such as with apo A-I infusions and upregulators of endogenous apo A-I production; indirectly augmenting apo A-I and HDL-cholesterol levels, such as through inhibition of cholesteryl ester transfer protein or endothelial lipase, or through activation of the high-affinity niacin receptor GPR109A; mimicking the functionality of apo A-I with apo A-I mimetic peptides; and enhancing steps in the reverse cholesterol transport pathway, such as via activation of the liver X receptor or of lecithin–cholesterol acyltransferase.
Key Points
-
Four HDL-targeted drug approaches exist: directly augmenting apolipoprotein A-I (apo A-I) levels, indirectly augmenting apo A-I and HDL-cholesterol levels, mimicking the effects of apo A-I, and enhancing reverse cholesterol transport
-
From a pharmacodynamic standpoint, direct augmentation of lipid-poor apo A-I levels arguably represents the most validated HDL therapeutic approach in terms of antiatherogenic potential
-
The clinical efficacy of interventions that raise HDL-cholesterol levels through slowing its metabolism, such as inhibition of cholesteryl ester transfer protein or endothelial lipase, remains to be established
-
The discovery of the niacin receptor GPR109A helped to define the mechanisms underlying the effect of niacin on free fatty acids and flushing, although it is unclear how niacin raises HDL-cholesterol levels
-
With the exception of D-4F, apo A-I mimetic peptides require parenteral administration and, therefore, are likely to be initially targeted to patients at high risk with acute coronary syndromes
-
Intestinal-specific liver X receptor (LXR) agonism or LXRβ-specific agonism might enhance reverse cholesterol transport while avoiding toxicity associated with nonselective LXR activation, namely, hepatic lipogenesis and hypertriglyceridemia
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Boden, W. E. et al. Optimal medical therapy with or without PCI for stable coronary disease. N. Engl. J. Med. 356, 1503–1516 (2007).
Frye, R. L. et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N. Engl. J. Med. 360, 2503–2515 (2009).
deGoma, E. M., deGoma, R. L. & Rader, D. J. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J. Am. Coll. Cardiol. 51, 2199–2211 (2008).
Wise, A. et al. Molecular identification of high and low affinity receptors for nicotinic acid. J. Biol. Chem. 278, 9869–9874 (2003).
Zhang, Y. et al. Overexpression of apolipoprotein A-I promotes reverse transport of cholesterol from macrophages to feces in vivo. Circulation 108, 661–663 (2003).
Badimon, J. J., Badimon, L. & Fuster, V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J. Clin. Invest. 85, 1234–1241 (1990).
Chiesa, G. et al. Recombinant apolipoprotein A-I(Milano) infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ. Res. 90, 974–980 (2002).
Parolini, C. et al. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: in vivo assessment by intravascular ultrasound and magnetic resonance imaging. J. Am. Coll. Cardiol. 51, 1098–1103 (2008).
Badimon, J. J., Badimon, L., Galvez, A., Dische, R. & Fuster, V. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab. Invest. 60, 455–461 (1989).
Shah, P. K. et al. Effects of recombinant apolipoprotein A-I(Milano) on aortic atherosclerosis in apolipoprotein E-deficient mice. Circulation 97, 780–785 (1998).
Barter, P. J. et al. Antiinflammatory properties of HDL. Circ. Res. 95, 764–772 (2004).
Dimayuga, P. et al. Reconstituted HDL containing human apolipoprotein A-1 reduces VCAM-1 expression and neointima formation following periadventitial cuff-induced carotid injury in apoE null mice. Biochem. Biophys. Res. Commun. 264, 465–468 (1999).
Nicholls, S. J. et al. Reconstituted high-density lipoproteins inhibit the acute pro-oxidant and proinflammatory vascular changes induced by a periarterial collar in normocholesterolemic rabbits. Circulation 111, 1543–1550 (2005).
Nissen, S. E. et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 290, 2292–2300 (2003).
Nissen, S. E. et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA 291, 1071–1080 (2004).
Tardif, J. C. et al. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA 297, 1675–1682 (2007).
Waksman, R. et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 55, 2727–2735 (2010).
Sirtori, C. R. et al. Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the Limone sul Garda study. Circulation 103, 1949–1954 (2001).
Ameli, S. et al. Recombinant apolipoprotein A-I Milano reduces intimal thickening after balloon injury in hypercholesterolemic rabbits. Circulation 90, 1935–1941 (1994).
Shah, P. K. et al. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation 103, 3047–3050 (2001).
Franceschini, G. et al. Increased cholesterol efflux potential of sera from ApoA-IMilano carriers and transgenic mice. Arterioscler. Thromb. Vasc. Biol. 19, 1257–1262 (1999).
Wang, L. et al. Bone marrow transplantation shows superior atheroprotective effects of gene therapy with apolipoprotein A-I Milano compared with wild-type apolipoprotein A-I in hyperlipidemic mice. J. Am. Coll. Cardiol. 48, 1459–1468 (2006).
Weibel, G. L. et al. Wild-type ApoA-I and the Milano variant have similar abilities to stimulate cellular lipid mobilization and efflux. Arterioscler. Thromb. Vasc. Biol. 27, 2022–2029 (2007).
Lebherz, C., Sanmiguel, J., Wilson, J. M. & Rader, D. J. Gene transfer of wild-type apoA-I and apoA-I Milano reduce atherosclerosis to a similar extent. Cardiovasc. Diabetol. 6, 15 (2007).
Parolini, C. et al. Apolipoprotein A-I and the molecular variant apoA-I(Milano): evaluation of the antiatherogenic effects in knock-in mouse model. Atherosclerosis 183, 222–229 (2005).
Alexander, E. T. et al. Macrophage reverse cholesterol transport in mice expressing ApoA-I Milano. Arterioscler. Thromb. Vasc. Biol. 29, 1496–1501 (2009).
Tardif, J. C. Emerging high-density lipoprotein infusion therapies: fulfilling the promise of epidemiology? J. Clin. Lipidol. 4, 399–404 (2010).
Lerch, P. G., Förtsch, V., Hodler, G. & Bolli, R. Production and characterization of a reconstituted high density lipoprotein for therapeutic applications. Vox Sang. 71, 155–164 (1996).
Mack, W. J., Xiang, M., Selzer, R. H. & Hodis, H. N. Serial quantitative coronary angiography and coronary events. Am. Heart J. 139, 993–999 (2000).
Jukema, J. W. et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS). Circulation 91, 2528–2540 (1995).
Waters, D. et al. Effects of monotherapy with an HMG-CoA reductase inhibitor on the progression of coronary atherosclerosis as assessed by serial quantitative arteriography. The Canadian Coronary Atherosclerosis Intervention Trial. Circulation 89, 959–968 (1994).
Shaw, J. A. et al. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ. Res. 103, 1084–1091 (2008).
Patel, S. et al. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J. Am. Coll. Cardiol. 53, 962–971 (2009).
Calkin, A. C. et al. Reconstituted high-density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux. Circulation 120, 2095–2104 (2009).
CSL Behring Research & Development Briefing [online], (2009).
Sacks, F. M. et al. Selective delipidation of plasma HDL enhances reverse cholesterol transport in vivo. J. Lipid Res. 50, 894–907 (2009).
Bailey, D. et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J. Am. Coll. Cardiol. 55, 2580–2589 (2010).
Miller, R. Oral inducer of apoA-1 synthesis RVX-208 misses end point but provides reasons for optimism [online], (2010).
Neeli, H. & Rader, D. J. Cholesteryl ester transfer protein (CETP) inhibitors: is there life after torcetrapib? Cardiol. Clin. 26, 537–546 (2008).
Boekholdt, S. M. et al. Plasma levels of cholesteryl ester transfer protein and the risk of future coronary artery disease in apparently healthy men and women: the prospective EPIC (European Prospective Investigation into Cancer and nutrition)-Norfolk population study. Circulation 110, 1418–1423 (2004).
Brousseau, M. E. et al. Cholesteryl ester transfer protein TaqI B2B2 genotype is associated with higher HDL cholesterol levels and lower risk of coronary heart disease end points in men with HDL deficiency: Veterans Affairs HDL Cholesterol Intervention Trial. Arterioscler. Thromb. Vasc. Biol. 22, 1148–1154 (2002).
Lu, B. et al. Causes of interscan variability of coronary artery calcium measurements at electron-beam CT. Acad. Radiol. 9, 654–661 (2002).
Ordovas, J. M. et al. Association of cholesteryl ester transfer protein-TaqIB polymorphism with variations in lipoprotein subclasses and coronary heart disease risk: the Framingham study. Arterioscler. Thromb. Vasc. Biol. 20, 1323–1329 (2000).
Thompson, A. et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA 299, 2777–2788 (2008).
Borggreve, S. E. et al. High plasma cholesteryl ester transfer protein levels may favour reduced incidence of cardiovascular events in men with low triglycerides. Eur. Heart J. 28, 1012–1018 (2007).
Borggreve, S. E. et al. An increased coronary risk is paradoxically associated with common cholesteryl ester transfer protein gene variations that relate to higher high-density lipoprotein cholesterol: a population-based study. J. Clin. Endocrinol. Metab. 91, 3382–3388 (2006).
Hirano, K. et al. Genetic cholesteryl ester transfer protein deficiency is extremely frequent in the Omagari area of Japan. Marked hyperalphalipoproteinemia caused by CETP gene mutation is not associated with longevity. Arterioscler. Thromb. Vasc. Biol. 17, 1053–1059 (1997).
Gaofu, Q. et al. Vaccinating rabbits with a cholesteryl ester transfer protein (CETP) B-Cell epitope carried by heat shock protein-65 (HSP65) for inducing anti-CETP antibodies and reducing aortic lesions in vivo. J. Cardiovasc. Pharmacol. 45, 591–598 (2005).
Morehouse, L. A. et al. Inhibition of CETP activity by torcetrapib reduces susceptibility to diet-induced atherosclerosis in New Zealand White rabbits. J. Lipid Res. 48, 1263–1272 (2007).
Okamoto, H. et al. A cholesteryl ester transfer protein inhibitor attenuates atherosclerosis in rabbits. Nature 406, 203–207 (2000).
Rittershaus, C. W. et al. Vaccine-induced antibodies inhibit CETP activity in vivo and reduce aortic lesions in a rabbit model of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 20, 2106–2112 (2000).
Sugano, M. et al. Effect of antisense oligonucleotides against cholesteryl ester transfer protein on the development of atherosclerosis in cholesterol-fed rabbits. J. Biol. Chem. 273, 5033–5036 (1998).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122 (2007).
Bots, M. L. et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet 370, 153–160 (2007).
Nicholls, S. J., Tuzcu, E. M., Brennan, D. M., Tardif, J. C. & Nissen, S. E. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation 118, 2506–2514 (2008).
Rader, D. J. Illuminating HDL—is it still a viable therapeutic target? N. Engl. J. Med. 357, 2180–2183 (2007).
Barter, P. Lessons learned from the Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events (ILLUMINATE) trial. Am. J. Cardiol. 104 (10 Suppl.), 10E–15E (2009).
Matsuura, F., Wang, N., Chen, W., Jiang, X. C. & Tall, A. R. HDL from CETP-deficient subjects shows enhanced ability to promote cholesterol efflux from macrophages in an apoE- and ABCG1-dependent pathway. J. Clin. Invest. 116, 1435–1442 (2006).
Forrest, M. J. et al. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br. J. Pharmacol. 154, 1465–1473 (2008).
Ishigami, M. et al. Large and cholesteryl ester-rich high-density lipoproteins in cholesteryl ester transfer protein (CETP) deficiency can not protect macrophages from cholesterol accumulation induced by acetylated low-density lipoproteins. J. Biochem. 116, 257–262 (1994).
Yvan-Charvet, L. et al. Inhibition of cholesteryl ester transfer protein by torcetrapib modestly increases macrophage cholesterol efflux to HDL. Arterioscler. Thromb. Vasc. Biol. 27, 1132–1138 (2007).
Yvan-Charvet, L. et al. Cholesterol efflux potential and antiinflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler. Thromb. Vasc. Biol. 30, 1430–1438 (2010).
Tanigawa, H. et al. Expression of cholesteryl ester transfer protein in mice promotes macrophage reverse cholesterol transport. Circulation 116, 1267–1273 (2007).
Vergeer, M. & Stroes, E. S. The pharmacology and off-target effects of some cholesterol ester transfer protein inhibitors. Am. J. Cardiol. 104 (10 Suppl.), 32E–38E (2009).
Niesor, E. J. et al. Modulating cholesteryl ester transfer protein activity maintains efficient pre-β-HDL formation and increases reverse cholesterol transport. J. Lipid Res. 51, 3443–3454 (2010).
de Grooth, G. J. et al. Efficacy and safety of a novel cholesteryl ester transfer protein inhibitor, JTT-705, in humans: a randomized phase II dose-response study. Circulation 105, 2159–2165 (2002).
Kuivenhoven, J. A. et al. Effectiveness of inhibition of cholesteryl ester transfer protein by JTT-705 in combination with pravastatin in type II dyslipidemia. Am. J. Cardiol. 95, 1085–1088 (2005).
Stein, E. A. et al. Safety and tolerability of dalcetrapib (RO4607381/JTT-705): results from a 48-week trial. Eur. Heart J. 31, 480–488 (2010).
ClinicalTrials.gov. A study of the effect of dalcetrapib on artherosclerotic disease in patients with coronary artery disease [online], (2010).
ClinicalTrials.gov. A study of the effect of RO4607381 on atherosclerotic plaque in patients with coronary heart disease [online], (2010).
ClinicalTrials.gov. A study assessing the effect of RO4607381 on vascular function in patients with coronary heart disease (CHD) or CHD-risk equivalent patients [online], (2010).
ClinicalTrials.gov. A study of RO4607381 in stable coronary heart disease patients with recent acute coronary syndrome [online], (2010).
Ranalletta, M. et al. Biochemical characterization of cholesteryl ester transfer protein inhibitors. J. Lipid Res. 51, 2739–2752 (2010).
Krishna, R. et al. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomised placebo-controlled phase I studies. Lancet 370, 1907–1914 (2007).
Bloomfield, D. et al. Efficacy and safety of the cholesteryl ester transfer protein inhibitor anacetrapib as monotherapy and coadministered with atorvastatin in dyslipidemic patients. Am. Heart J. 157, 352–360 (2009).
Cannon, C. P. et al. Design of the DEFINE trial: determining the EFficacy and tolerability of CETP INhibition with AnacEtrapib. Am. Heart J. 158, 513–519 (2009).
Cannon, C. P. et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N. Engl. J. Med. 363, 2406–2415 (2010).
ClinicalTrials.gov. REVEAL: Randomized EValuation of the Effects of Anacetrapib through Lipid-modification [online], (2010).
Taylor, A. J., Sullenberger, L. E., Lee, H. J., Lee, J. K. & Grace, K. A. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 110, 3512–3517 (2004).
Brown, B. G. et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 345, 1583–1592 (2001).
Canner, P. L. et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J. Am. Coll. Cardiol. 8, 1245–1255 (1986).
Villines, T. C. et al. The ARBITER 6-HALTS Trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis): final results and the impact of medication adherence, dose, and treatment duration. J. Am. Coll. Cardiol. 55, 2721–2726 (2010).
ClinicalTrials.gov. Niacin plus statin to prevent vascular events [online], (2010).
ClinicalTrials.gov. Treatment of HDL to reduce the incidence of vascular events HPS2-THRIVE [online], (2010).
Altschul, R., Hoffer, A. & Stephen, J. D. Influence of nicotinic acid on serum cholesterol in man. Arch. Biochem. 54, 558–559 (1955).
Jin, F. Y., Kamanna, V. S. & Kashyap, M. L. Niacin decreases removal of high-density lipoprotein apolipoprotein A-I but not cholesterol ester by Hep G2 cells. Implication for reverse cholesterol transport. Arterioscler. Thromb. Vasc. Biol. 17, 2020–2028 (1997).
Shepherd, J., Packard, C. J., Patsch, J. R., Gotto, A. M. Jr & Taunton, O. D. Effects of nicotinic acid therapy on plasma high density lipoprotein subfraction distribution and composition and on apolipoprotein A metabolism. J. Clin. Invest. 63, 858–867 (1979).
Blum, C. B. et al. High density lipoprotein metabolism in man. J. Clin. Invest. 60, 795–807 (1977).
Lamon-Fava, S. et al. Extended-release niacin alters the metabolism of plasma apolipoprotein (Apo) A-I and ApoB-containing lipoproteins. Arterioscler. Thromb. Vasc. Biol. 28, 1672–1678 (2008).
Tunaru, S. et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9, 352–355 (2003).
Maccubbin, D. et al. Flushing profile of extended-release niacin/laropiprant versus gradually titrated niacin extended-release in patients with dyslipidemia with and without ischemic cardiovascular disease. Am. J. Cardiol. 104, 74–81 (2009).
Hanson, J. et al. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J. Clin. Invest. 120, 2910–2919 (2010).
Dunbar, R. L. & Gelfand, J. M. Seeing red: flushing out instigators of niacin-associated skin toxicity. J. Clin. Invest. 120, 2651–2655 (2010).
Richman, J. G. et al. Nicotinic acid receptor agonists differentially activate downstream effectors. J. Biol. Chem. 282, 18028–18036 (2007).
Lai, E. et al. Effects of a niacin receptor partial agonist, MK-0354, on plasma free fatty acids, lipids, and cutaneous flushing in humans. J. Clin. Lipidol. 2, 375–383 (2008).
Choi, S. Y., Hirata, K., Ishida, T., Quertermous, T. & Cooper, A. D. Endothelial lipase: a new lipase on the block. J. Lipid Res. 43, 1763–1769 (2002).
Ishida, T. et al. Endothelial lipase is a major determinant of HDL level. J. Clin. Invest. 111, 347–355 (2003).
Jin, W., Millar, J. S., Broedl, U., Glick, J. M. & Rader, D. J. Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo. J. Clin. Invest. 111, 357–362 (2003).
Jaye, M. et al. A novel endothelial-derived lipase that modulates HDL metabolism. Nat. Genet. 21, 424–428 (1999).
Edmondson, A. C. et al. Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J. Clin. Invest. 119, 1042–1050 (2009).
Badellino, K. O., Wolfe, M. L., Reilly, M. P. & Rader, D. J. Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PLoS Med. 3, e22 (2006).
Badellino, K. O., Wolfe, M. L., Reilly, M. P. & Rader, D. J. Endothelial lipase is increased in vivo by inflammation in humans. Circulation 117, 678–685 (2008).
Tang, N. P. et al. Protective effect of an endothelial lipase gene variant on coronary artery disease in a Chinese population. J. Lipid Res. 49, 369–375 (2008).
Vergeer, M. et al. Lack of association between common genetic variation in endothelial lipase (LIPG) and the risk for CAD and DVT. Atherosclerosis 211, 558–564 (2010).
Jensen, M. K. et al. The T111I variant in the endothelial lipase gene and risk of coronary heart disease in three independent populations. Eur. Heart J. 30, 1584–1589 (2009).
Brown, R. J. et al. Impact of combined deficiency of hepatic lipase and endothelial lipase on the metabolism of both high-density lipoproteins and apolipoprotein B-containing lipoproteins. Circ. Res. 107, 357–364 (2010).
Goodman, K. B. et al. Discovery of potent, selective sulfonylfuran urea endothelial lipase inhibitors. Bioorg. Med. Chem. Lett. 19, 27–30 (2009).
Van Lenten, B. J. et al. Apolipoprotein A-I mimetic peptides. Curr. Atheroscler. Rep. 11, 52–57 (2009).
Navab, M. et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J. Lipid Res. 45, 993–1007 (2004).
Anantharamaiah, G. M. et al. Structural requirements for antioxidative and anti-inflammatory properties of apolipoprotein A-I mimetic peptides. J. Lipid Res. 48, 1915–1923 (2007).
Navab, M. et al. Oral administration of an Apo A-I mimetic Peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation 105, 290–292 (2002).
Datta, G. et al. Effects of increasing hydrophobicity on the physical-chemical and biological properties of a class A amphipathic helical peptide. J. Lipid Res. 42, 1096–1104 (2001).
Smythies, L. E. et al. Apolipoprotein A-I mimetic 4F alters the function of human monocyte-derived macrophages. Am. J. Physiol. Cell Physiol. 298, C1538–C1548 (2010).
Song, X., Fischer, P., Chen, X., Burton, C. & Wang, J. An apoA-I mimetic peptide facilitates off-loading cholesterol from HDL to liver cells through scavenger receptor BI. Int. J. Biol. Sci. 5, 637–646 (2009).
Van Lenten, B. J. et al. Anti-inflammatory apoA-I-mimetic peptides bind oxidized lipids with much higher affinity than human apoA-I. J. Lipid Res. 49, 2302–2311 (2008).
Xie, Q., Zhao, S. P. & Li, F. D-4F, an apolipoprotein A-I mimetic peptide, promotes cholesterol efflux from macrophages via ATP-binding cassette transporter A1. Tohoku J. Exp. Med. 220, 223–228 (2010).
Buga, G. M. et al. L-4F alters hyperlipidemic (but not healthy) mouse plasma to reduce platelet aggregation. Arterioscler. Thromb. Vasc. Biol. 30, 283–289 (2010).
Li, X. et al. Differential effects of apolipoprotein A-I-mimetic peptide on evolving and established atherosclerosis in apolipoprotein E-null mice. Circulation 110, 1701–1705 (2004).
Navab, M. et al. D-4F and statins synergize to render HDL antiinflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 25, 1426–1432 (2005).
Bloedon, L. T. et al. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J. Lipid Res. 49, 1344–1352 (2008).
Rader, D. J. Molecular regulation of HDL metabolism and function: implications for novel therapies. J. Clin. Invest. 116, 3090–3100 (2006).
Ruchala, P. et al. Oxpholipin 11D: an anti-inflammatory peptide that binds cholesterol and oxidized phospholipids. PLoS One 5, e10181 (2010).
Bielicki, J. K. et al. A new HDL mimetic peptide that stimulates cellular cholesterol efflux with high efficiency greatly reduces atherosclerosis in mice. J. Lipid Res. 51, 1496–1503 (2010).
D'Souza, W. et al. Structure/function relationships of apolipoprotein a-I mimetic peptides: implications for antiatherogenic activities of high-density lipoprotein. Circ. Res. 107, 217–227 (2010).
Rader, D. J. Liver X receptor and farnesoid X receptor as therapeutic targets. Am. J. Cardiol. 100 (Suppl. 1), S15–S19 (2007).
Rigamonti, E. et al. Liver X receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circ. Res. 97, 682–689 (2005).
Costet, P., Luo, Y., Wang, N. & Tall, A. R. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 275, 28240–28245 (2000).
Brunham, L. R. et al. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J. Clin. Invest. 116, 1052–1062 (2006).
Alberti, S., Steffensen, K. R. & Gustafsson, J. A. Structural characterisation of the mouse nuclear oxysterol receptor genes LXRα and LXRβ. Gene 243, 93–103 (2000).
Repa, J. J. et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRα and LXRβ. Genes Dev. 14, 2819–2830 (2000).
Grefhorst, A. et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 277, 34182–34190 (2002).
Peet, D. J. et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell 93, 693–704 (1998).
Alberti, S. et al. Hepatic cholesterol metabolism and resistance to dietary cholesterol in LXRβ-deficient mice. J. Clin. Invest. 107, 565–573 (2001).
Lund, E. G. et al. Different roles of liver X receptor α and β in lipid metabolism: effects of an α-selective and a dual agonist in mice deficient in each subtype. Biochem. Pharmacol. 71, 453–463 (2006).
Quinet, E. M. et al. Liver X receptor (LXR)-β regulation in LXRα-deficient mice: implications for therapeutic targeting. Mol. Pharmacol. 70, 1340–1349 (2006).
Bradley, M. N. et al. Ligand activation of LXRβ reverses atherosclerosis and cellular cholesterol overload in mice lacking LXRα and apoE. J. Clin. Invest. 117, 2337–2346 (2007).
Fiévet, C. & Staels, B. Liver X receptor modulators: effects on lipid metabolism and potential use in the treatment of atherosclerosis. Biochem. Pharmacol. 77, 1316–1327 (2009).
Yoshikawa, T. et al. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol. Cell. Biol. 21, 2991–3000 (2001).
Inaba, T. et al. Angiopoietin-like protein 3 mediates hypertriglyceridemia induced by the liver X receptor. J. Biol. Chem. 278, 21344–21351 (2003).
Jakel, H. et al. The liver X receptor ligand T0901317 down-regulates APOA5 gene expression through activation of SREBP-1c. J. Biol. Chem. 279, 45462–45469 (2004).
Schaap, F. G. et al. ApoAV reduces plasma triglycerides by inhibiting very low density lipoprotein-triglyceride (VLDL-TG) production and stimulating lipoprotein lipase-mediated VLDL-TG hydrolysis. J. Biol. Chem. 279, 27941–27947 (2004).
Yasuda, T. et al. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler. Thromb. Vasc. Biol. 30, 781–786 (2010).
Lo Sasso, G. et al. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell. Metab. 12, 187–193 (2010).
Föger, B. et al. Cholesteryl ester transfer protein corrects dysfunctional high density lipoproteins and reduces aortic atherosclerosis in lecithin cholesterol acyltransferase transgenic mice. J. Biol. Chem. 274, 36912–36920 (1999).
Hoeg, J. M. et al. Overexpression of lecithin:cholesterol acyltransferase in transgenic rabbits prevents diet-induced atherosclerosis. Proc. Natl Acad. Sci. USA 93, 11448–11453 (1996).
Mertens, A. et al. Increased low-density lipoprotein oxidation and impaired high-density lipoprotein antioxidant defense are associated with increased macrophage homing and atherosclerosis in dyslipidemic obese mice: LCAT gene transfer decreases atherosclerosis. Circulation 107, 1640–1646 (2003).
Santamarina-Fojo, S., Lambert, G., Hoeg, J. M. & Brewer, H. B. Jr. Lecithin-cholesterol acyltransferase: role in lipoprotein metabolism, reverse cholesterol transport and atherosclerosis. Curr. Opin. Lipidol. 11, 267–275 (2000).
Rader, D. J. Lecithin:cholesterol acyltransferase and atherosclerosis. Another high-density lipoprotein story that doesn't quite follow the script. Circulation 120, 549–552 (2009).
Calabresi, L. et al. Functional lecithin:cholesterol acyltransferase is not required for efficient atheroprotection in humans. Circulation 120, 628–635 (2009).
Holleboom, A. G. et al. Plasma levels of lecithin:cholesterol acyltransferase and risk of future coronary artery disease in apparently healthy men and women: a prospective case-control analysis nested in the EPIC-Norfolk population study. J. Lipid Res. 51, 416–421 (2010).
Hovingh, G. K. et al. Compromised LCAT function is associated with increased atherosclerosis. Circulation 112, 879–884 (2005).
Dullaart, R. P., Perton, F., van der Klauw, M. M., Hillege, H. L. & Sluiter, W. J. High plasma lecithin:cholesterol acyltransferase activity does not predict low incidence of cardiovascular events: possible attenuation of cardioprotection associated with high HDL cholesterol. Atherosclerosis 208, 537–542 (2010).
Tanigawa, H. et al. Lecithin:cholesterol acyltransferase expression has minimal effects on macrophage reverse cholesterol transport in vivo. Circulation 120, 160–169 (2009).
Acknowledgements
This work was supported by grants from the National Heart, Lung and Blood Institute (HL22633 and P50 HL70128) and the National Center for Research Resources (UL1-RR-024134). E. M. deGoma's salary is partially funded by a National Heart, Lung and Blood Institute grant (K12 HL083772-01).
Author information
Authors and Affiliations
Contributions
E. M. deGoma and D. J. Rader contributed equally to the discussion of content for the article, the research of data to include in the manuscript, writing the article and the reviewing and editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
D. J. Rader is a consultant for Abbott, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, Novartis, and Resverlogix. E. M. deGoma declares no competing interests.
Rights and permissions
About this article
Cite this article
deGoma, E., Rader, D. Novel HDL-directed pharmacotherapeutic strategies. Nat Rev Cardiol 8, 266–277 (2011). https://doi.org/10.1038/nrcardio.2010.200
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2010.200
This article is cited by
-
Potential role of new molecular plasma signatures on cardiovascular risk stratification in asymptomatic individuals
Scientific Reports (2018)
-
Efficacy and safety assessment of a TRAF6-targeted nanoimmunotherapy in atherosclerotic mice and non-human primates
Nature Biomedical Engineering (2018)
-
A common functional promoter variant links CNR1 gene expression to HDL cholesterol level
Nature Communications (2013)
-
HDL—is it too big to fail?
Nature Reviews Endocrinology (2013)
-
HDL – „Game over“?
Der Kardiologe (2013)