Abstract
Background:
The aim of this study was to assess the value of N-terminal pro-brain natriuretic peptide (NT-proBNP) in predicting late cardiotoxicity in patients treated with not-high-dose chemotherapy (NHDC), and to compare the predictive value of NT-proBNP and cardiac troponin I (cTnI).
Methods:
In 71 patients undergoing NHDC with anthracyclines, NT-proBNP and cTnI levels were measured before and 24 h after each NHDC cycle. Left ventricular (LV) function was assessed by echocardiography at baseline, every two NHDC cycles, at the end of chemotherapy, and at 3-, 6- and 12-month follow-up.
Results:
During NHDC, only NT-proBNP showed abnormal values. According to NT-proBNP behaviour, patients were divided into two groups: group A (n=50) with normal (n=23) or transiently elevated NT-proBNP levels (n=27), and group B (n=21) with persistently elevated NT-proBNP levels. At follow-up, LV impairment was significantly worse in group B than in group A. %Δ (baseline–peak) NT-proBNP was predictive of LV impairment at 3-, 6- and 12-month follow-up, with a cutoff of 36%.
Conclusion:
Serial measurements of NT-proBNP may be a useful tool for the early detection of patients treated with NHDC at high risk of developing cardiotoxicity.
Similar content being viewed by others
Main
Anthracyclines are powerful chemotherapy agents that are widely used for the treatment of solid and haematological malignancies in both adults and children. Their introduction has led to the successful treatment of several forms of cancer and contributed to reducing mortality and increasing long-term survival.
Notwithstanding this, the use of anthracyclines is limited by dose-dependent cardiotoxicity (Shan et al, 1996; Singal and Iliskovic, 1998; Hequet et al, 2004; Yeh and Bickford, 2009). Several patients show early or long-term cardiac abnormalities, such as impaired left ventricular (LV) function and/or cardiomyopathy, which may result in overt heart failure and cardiac death. In a series of >5000 long-term cancer survivors treated with chemotherapy agents, the probability of death after relapse was the same as after cardiac complications due to chemotherapy (Schultz et al, 2003).
Left ventricular ejection fraction (LVEF), as assessed by echocardiography, is the most commonly used parameter to evaluate LV function in cancer patients undergoing chemotherapy. However, LVEF measurements are not sensitive for the early detection of pre-clinical cardiac disease and are affected by changes in contractility, preload and afterload (Pai and Nahata, 2000; Gharib and Burnett, 2002; Yeh and Bickford, 2009). This emphasises the need for non-invasive, highly sensitive and inexpensive diagnostic tools for the early identification of high-risk patients.
Several studies suggested that cardiac biomarkers may be useful for the identification of patients at high risk of developing cardiotoxicity. The most robust results were obtained using cardiac troponin I (cTnI) (Cardinale et al, 2000, 2004; Sparano et al, 2002; Adamcova et al, 2005; Kilickap et al, 2005), whereas the role of natriuretic peptides remains less clear (Bauch et al, 1992; Suzuki et al, 1998; Meinardi et al, 2001, 2002; Sandri et al, 2005; Germanakis et al, 2006; Kouloubinis et al, 2007).
The majority of studies included inpatient cohorts undergoing high-dose chemotherapy (HDC), where blood samples for the determination of cardiac markers can be collected more easily. Only few studies reported the measurement of cardiac markers in outpatients undergoing not-high-dose chemotherapy (NHDC). Although this subset of patients represents the vast majority in several varieties of cancer, information is lacking about the value of cardiac markers as predictors of LV dysfunction.
The aim of our study was (i) to assess N-terminal pro-brain natriuretic peptide (NT-proBNP) increase and behaviour in predicting late cardiotoxicity in patients treated with NHDC and (ii) to compare the predictive value of NT-proBNP and cTnI, the latter being a well-established predictor of cardiotoxicity.
Materials and methods
Study population
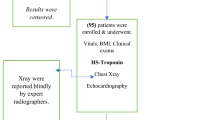
Ninety-two consecutive breast cancer patients undergoing NHDC with anthracyclines from February 2007 to February 2008 were initially screened for this study. Study eligibility criteria were age between 18 and 75 years, a good performance status (⩽2 on ZUBROD-ECOG-WHO scale), serum creatinine <1.5 mg dl–1, normal hepatic function. The following exclusion criteria were applied: history of coronary artery disease, haemodynamically significant valvular heart disease, LVEF <50%, immunological diseases or other serious diseases limiting life expectancy (decompensated diabetes, renal failure, etc.). Patients who had received chemotherapy or local radiotherapy before or during the study period were also excluded. Eleven patients did not match the inclusion/exclusion criteria. In addition, seven patients were excluded because of poor echocardiographic image quality rendering evaluation of LV function unfeasible, and three patients were excluded because of drop-out after the first or second blood draw. Therefore, the final study population consisted of 71 patients, of which 27 had metastatic breast cancer.
Thirty-four patients (48%) were treated with liposome-encapsulated doxorubicin (40–50 mg m–2) and docetaxel (50 mg m–2) for 6 cycles (every 14 days, dose-dense protocol), and 37 patients (52%) were treated with epirubicin (90 mg m–2) in combination with fluorouracil and cyclophosphamide (FEC protocol) for 6 cycles (every 21 days). Patients treated with liposome-encapsulated doxorubicin received a median cumulative doxorubicin dose 300 mg m–2. Among FEC-treated patients, dose intensity of epirubicin was >80% and up to 540 mg m–2 cumulative dose.
All patients gave their written informed consent. The investigation conformed with the principles outlined in the Declaration of Helsinki and was approved by the local ethics committee.
Echocardiography
Echocardiographic examination was performed with a commercially available imaging system (MyLab 30, EsaoteBiomedica, Genoa, Italy). Data acquisition was performed with a 3.5-MHz transducer in the parasternal and apical views (standard 2- and 4-chamber images). Two-dimensional and Doppler images were obtained during breath hold and stored in cineloop format from three consecutive beats; then the average values of LV volumes and LVEF were reported.
LV end-diastolic (LVEDV) and end-systolic volumes (LVESV) and LVEF were derived from the apical biplane modified Simpson's rule algorithm. All echocardiograms were interpreted by two experienced cardiologists who were blinded to the patient history and laboratory results; inter-observer and intra-observer variability were 8.7% and 7.9%, respectively.
Laboratory examination
Blood samples were taken after 5 min of supine rest. NT-proBNP was measured using the commercially available Elecsys proBNP sandwich immunoassay on an Elecsys 2010 (Roche Diagnostics, Mannheim, Germany) for quantitative determination of the peptide in serum and plasma. Intra- and inter-assay variability coefficients were 2.5% and 3.1%, respectively. The upper reference limits of NT-proBNP were 153 ng l–1 for patients aged ⩽50 years and 222 ng l–1 for patients aged >50 years, according to the manufacturer's guidelines. cTnI concentrations were determined by a fluorometric enzyme immunoassay analyser (Stratus CS Acute Care, Dade Behring, Deerfield, IL, USA); the cutoff levels were 5 and 0.08 ng ml–1, respectively. All positive samples were immediately retested for confirmation.
Study protocol
All patients underwent complete anamnestic, clinical, ECG, echocardiographic and laboratory evaluation 1 week before starting chemotherapy to verify the inclusion/exclusion criteria. cTnI and NT-proBNP samples were collected from enrolled patients before and 24 h after each drug administration. Echocardiographic evaluation was performed at baseline (1 week before starting chemotherapy), every 2 cycles, at the end of chemotherapy, and at 3-, 6- and 12-month follow-up (Figure 1).
The increase in NT-proBNP was defined as transient when elevated NT-proBNP levels decreased within the normal range at repeat measurements during NHDC, and permanent when elevated NT-proBNP levels were recorded at each measurement.
End points and statistics
The primary end point for the sample size calculation was the difference between group A (no or transient NT-proBNP elevation) and group B (permanent NT-proBNP elevation) in the percentage change of LVEF from baseline to 1-year follow-up. In accordance with Sandri et al (2005), assuming an α-error of 0.05 and a power of 90%, the required sample size was 54 patients. Then, the relationship between permanent NT-proBNP elevation throughout NHDC cycles and structural/functional cardiac alterations, both during NHDC and after the end of drug administration, was evaluated. The same relationship was investigated for cTnI.
The percentage Δ (baseline–peak) NT-proBNP was obtained using the following formula: (baseline NT-proBNP−peak NT-proBNP/baseline NT-proBNP) × 100; the highest NT-proBNP measurement was recorded as the peak value reached during NHDC.
Results were expressed as mean±s.d. for normally distributed continuous variables, and as median and interquartile range (IQR; 25th and 75th percentile) for not normally distributed data. Categorical variables were reported as count and percentage. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. Paired and unpaired statistical comparisons were made using the Wilcoxon test and the Student's t-test or Mann–Whitney U-test, respectively. Categorical variables were compared using χ2 or Fisher exact test, where appropriate.
The NT-proBNP group, time of assessment, baseline NT-proBNP, peak NT-proBNP, %Δ NT-proBNP (baseline–peak), protocol of therapy, cumulative dose of anthracyclines, age, baseline LVEF, hypertension, diabetes, hypercholesterolemia, smokers, ACE-inhibitors, β-blockers, AT2-receptor blockers were initially tested by univariate analysis; those with a P-value of <0.1 were entered into a mixed linear regression model to assess whether LVEF impairment over time was dependent on the response pattern of NT-proBNP. Results were reported as β-coefficient±s.e. and P-value.
Receiver operating characteristic (ROC) curve analysis was used to identify any cutoff of %Δ NT-proBNP (baseline–peak) predictive of LV impairment from baseline to 3-, 6- and 12-month follow-up. %Δ (baseline–peak) NT-proBNP was entered into the ROC curve analysis as a continuous predictive variable. LV impairment from baseline to 3-, 6- and 12-month follow-up was used as a classification variable (target event). LV impairment was defined as a decrease in LVEF of ⩾20% and/or an increase in LVESV of ⩾15% from baseline (Mele et al, 2006).
Statistical significance was evaluated against 1000 bootstrap samples. A probability value of <0.05 was considered significant. All analyses were performed with the statistical software package SPSS (SPSS Inc., Chicago, IL, USA).
Results
Demographical, clinical and echocardiographic data and chemotherapy protocol did not differ significantly between groups (Table 1).
Biochemical measurements showed normal NT-proBNP and cTnI levels at baseline in all patients. During NHDC, cTnI was abnormal only occasionally in four patients. Conversely, elevated NT-proBNP levels were detected in many samples, and patients were divided into two groups according to changes in NT-proBNP concentrations: group A (n=50) showing normal (n=23) or transiently elevated NT-proBNP levels (n=27, increase at 24 h with subsequent normalisation) at repeated measurements, and group B (n=21) showing persistently elevated NT-proBNP levels at repeated measurements (Table 2). No difference was found between groups in the cumulative dose of anthracyclines.
Echocardiographic results
During NHDC, only two patients showed a >10% reduction in LVEF, but in both cases LVEF remained >50%. The magnitude of mean LVEF reduction across NHDC was 2% (IQR −0.25%; −4%) in group A and 4% (IQR 3%; 6%) in group B.
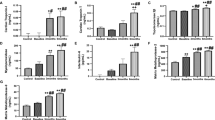
In group A, no significant differences in serial measurements of LVEF were observed, whereas group B showed a significant reduction in LVEF over time (Figure 2A), in particular 3 months after the end of NHDC administration. At mixed linear regression analysis, permanent NT-proBNP elevation during NHDC was correlated with LVEF reduction 12 months after the end of NHDC (β=22.8±9.5, P=0.013).
Regarding LV volumes, no differences were observed in group A in serial measurements compared with baseline; in group B LVEDV tended to increase significantly after the sixth NHDC cycle, whereas LVESV was found to increase by the second NHDC cycle (Figure 2).
Moreover, LVEF and LVESV were significantly different in group A and group B at 3-month follow-up and at the end of NHDC, respectively (Figure 2).
ROC curve analysis
Δ (baseline–peak) NT-proBNP was predictive of LV impairment at 3- (AUC=0.81, 95% CI=0.70–0.90), 6- (AUC=0.75, 95% CI=0.62–0.83) and 12-month follow-up (AUC=0.77, 95% CI=0.66–0.86) (Figure 3). A Δ (baseline–peak) NT-proBNP value higher than 36% was predictive of LV impairment at 3- (sensitivity 92.3%, specificity 65.2%), 6- (sensitivity 78.3%, specificity 70.8%) and 12-month follow-up (sensitivity 79.2%, specificity 72.3%) (Figure 3). Patients with persistent NT-proBNP elevation had a Δ (baseline–peak) NT-proBNP value higher than 36% vs just 24% in group A (P<0.001).
Clinical events
At 12-month follow-up, two patients experienced dyspnoea (after the end of chemotherapy) with clinical evidence of heart failure, and two additional patients developed supraventricular arrhythmias (during chemotherapy). It is worth noting that these four patients belonged to group B (with persistent NT-proBNP elevation).
Discussion
There is increasing evidence for the clinical relevance of chemotherapy-induced cardiotoxicity in cancer patients. The large number of cancer survivors increases the likelihood of developing LV dysfunction and overt heart failure. This is especially true in breast cancer patients who need to be treated with anthracyclines, the most cardiotoxic antiblastic drugs (Singal and Iliskovic, 1998; Hequet et al, 2004; Yeh and Bickford, 2009). In addition, female gender is a well-established risk factor for cardiotoxicity (Lipshultz et al, 1995).
Anthracycline-induced cardiotoxicity has traditionally been categorised into acute, early-onset chronic progressive, and late-onset chronic progressive. It leads predominantly to LV dysfunction and subsequent heart failure, often with dismal prognosis. In the clinical setting, monitoring of anthracycline-induced cardiotoxicity is usually performed by LVEF measurement, as assessed by echocardiography or radionuclide scintigraphy. LVEF depression represents a late marker of cardiotoxicity and allows the identification of patients with irreversible myocardial damage. The need for an early marker of cardiac injury is therefore undeniable, and several studies addressed this topic. In patients with aggressive malignancies treated with HDC, Cardinale et al (2000, 2004) demonstrated that cTnI accurately predicts future LVEF depression. This finding was confirmed in subsequent studies that highlighted the value of troponins in this patient subset (Auner et al, 2003; Lipshultz et al, 2004; Kilickap et al, 2005).
In recent years, several studies have suggested the use of BNP as a screening test for asymptomatic LV dysfunction (Latour-Pérez et al, 2006; Romano et al, 2009), and some authors have reported conflicting results regarding the value of BNP and NT-proBNP in cancer patients undergoing chemotherapy (Bauch et al, 1992; Suzuki et al, 1998; Meinardi et al, 2001, 2002; Sandri et al, 2005; Germanakis et al, 2006; Kouloubinis et al, 2007).
In our study, we evaluated breast cancer patients treated with chemotherapy regimens containing liposomal doxorubicin and epirubicin with a lower degree of cardiotoxic activity than doxorubicin. At present, this treatment is commonly used in breast cancer patients, and our results may have an important clinical impact. To the best of our knowledge, this is the first study evaluating serial determination of cTnI and NT-proBNP in this subset of patients.
Our results show a different response of NT-proBNP in patients undergoing anthracycline-based chemotherapy, with subsequent impairment of LV function only in patients with persistent NT-proBNP elevation. This finding makes the detection of NT-proBNP abnormalities very useful in the early identification of patients at high risk of developing LV dysfunction. As a matter of fact, the persistence of elevated NT-proBNP concentrations may reflect decreased contractile reserve. A recent review has investigated the potential mechanisms underlying anthracycline-induced cardiotoxicity (Sawyer et al, 2010). Among the proposed mechanisms, ‘sarcomere disruption’ seems to better support the hypothesis of reduced contractile reserve (increased BNP levels), even in the absence of cell death (normal troponin values) (Mortensen et al, 1986; Rowan et al, 1988; Mackay et al, 1994). Anthracycline exposure induces titin degradation: titin is a giant myofilament protein, which serves as a scaffold for assembly of myofilamentous proteins into sarcomeres. As a consequence, its integrity is essential for the dynamic regulation of contractile function. The loss of intact titin has been demonstrated in failing human hearts, implicating titin disruption in the pathogenesis of progressive ventricular dysfunction (Wang et al, 1988; Helmes et al, 1996, 2003).
Our results concerning the diagnostic value of cTnI are unexpected, as in the current literature, chemotherapy-induced cTnI elevation is reported in roughly 30% of patients undergoing HDC (Cardinale et al, 2000, 2004). Most studies focused on patients undergoing HDC, and only limited information is available about NHDC, leaving the debate open. In 100 consecutive patients receiving low-to-moderate doses of anthracyclines, Dodos et al (2008) failed to demonstrate any predictive value of either troponins or BNP. Feola et al (2011) did not find any differences in cTnI measured at baseline and 1 month after NHDC between patients developing cardiotoxicity and the remaining population.
Sawaya et al (2011) demonstrated that, after 3 months of treatment, elevated cTnI levels were observed in 28% of patients and were predictive of cardiotoxicity. However, they used a high-sensitivity assay that enabled detection of minimal marker elevation.
Our different findings may be due to two factors. On one hand, our study population was treated with lower doses of cytotoxic drugs, causing less myocardial injury. On the other, according to our study protocol, only one blood sample was scheduled after each chemotherapy cycle because all patients were outpatients. Conversely, previous studies on HDC usually enrolled inpatients, in whom serial blood samples were collected, increasing the likelihood of detecting cTnI elevation.
Comparison with previous studies on natriuretic peptides
Up to now, only few studies with a limited number of patients have evaluated the role of natriuretic peptides after chemotherapy in cancer patients.
Our results are consistent with those reported by Sandri et al (2005), who studied 52 patients treated with HDC for aggressive malignancies. They observed three different behaviours of NT-proBNP concentrations: (1) increased NT-proBNP levels up to 72 h after the end of chemotherapy in 17 patients; (2) initial elevated NT-proBNP levels with return to baseline values after 72 h in 19 patients; and (3) no NT-proBNP elevation in 16 patients. The evaluation of LV function showed that only persistently increased plasma NT-proBNP early after HDC was strongly associated with the development of cardiac dysfunction. Our results show a similar pattern of NT-proBNP changes in patients undergoing NHDC, with functional impairment more likely to occur in patients with persistently elevated NT-proBNP levels.
Some studies have evaluated natriuretic peptides in NHDC patients, showing elevated BNP or NT-proBNP levels in most subjects (Meinardi et al, 2001; Pichon et al, 2005; Kouloubinis et al, 2007; Feola et al, 2011). However, no assessment of their predictive value in identifying patients at high risk of developing LV dysfunction and/or heart failure was made at subsequent follow-up. In the study by Feola et al (2011), the patients who experienced cardiotoxicity showed elevated BNP levels 1 and 2 years after NHDC. However, no significant BNP changes were observed over time, which is more clinically relevant for the early identification of patients at high risk of developing LV dysfunction. Conversely, the results of our study demonstrate that NT-proBNP may serve as a useful marker for the early detection of such patients.
Study limitations
This study has some limitations that need to be considered. First, it is an observational study, not a randomised, controlled trial. Second, the collection of only one blood sample 24 h after each NHDC cycle may have led to a low number of ‘positive’ patients, especially cTnI-positive patients. Data from previous studies on HDC show that ∼20% of patients have abnormal values 36 or 72 h after treatment. Notwithstanding this, the results obtained with NT-proBNP are not affected by this limitation, because the most important finding is the return to baseline values in the sample collected before the subsequent cycle of chemotherapy (14 or 21 days later). In addition, we also enrolled patients with arterial hypertension, although this condition is known to cause NT-proBNP elevation. This may mask or reduce the negative effects of chemotherapy on LV volumes and function.
However, our inclusion/exclusion criteria allowed us to enrol a study population that was more representative of the ‘real world’. Our data showed normal NT-proBNP levels at baseline also in hypertensive patients, and no difference was present in the frequency of hypertension or antihypertensive drugs between the two groups of patients divided according to NT-proBNP behaviour.
Finally, no assessment of diastolic function was made, thus limiting the possibility of investigating whether cardiac biomarkers can predict diastolic impairment of LV function.
Clinical implications
Antineoplastic therapy is frequently complicated by the development of cardiotoxicity, which leads to cardiomyopathy evolving towards heart failure. In some instances, LV impairment is detected too late at echocardiography, when significant myocardial damage has already occurred. The availability of a biomarker for the identification of patients at high risk of developing cardiotoxicity would be of particular benefit, prompting oncologists and cardiologists to ensure close monitoring of these patients and to start adequate preventive or therapeutic management of LV dysfunction.
Serial measurements of NT-proBNP levels in NHDC patients may be even more useful than cTnI in the early identification of patients at high risk of developing anthracycline-induced cardiotoxicity. As recently demonstrated by Cardinale et al (2010), LVEF recovery in cancer patients undergoing anthracycline administration may be achieved if LV dysfunction is detected early and appropriate heart failure therapy is promptly initiated.
Further studies are warranted to test the possibility of reducing anthracycline-induced cardiotoxicity by adopting an NT-proBNP-guided preventive strategy.
Change history
29 March 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Adamcova M, Sterba M, Simunek T, Potacova A, Popelova O, Mazurova Y, Gersl V (2005) Troponin as a marker of myocardiac damage in drug-induced cardiotoxicity. Expert Opin Drug Saf 4: 457–472
Auner HW, Tinchon C, Linkesch W, Tiran A, Quehenberger F, Link H, Sill H (2003) Prolonged monitoring of troponin T for the detection of anthracycline cardiotoxicity in adults with hematological malignancies. Ann Hematol 82: 218–222
Bauch M, Ester A, Kimura B, Victorica BE, Kedar A, Phillips MI (1992) Atrial natriuretic peptide as a marker for doxorubicin-induced cardiotoxic effects. Cancer 69: 1492–1497
Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM (2010) Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 55: 213–220
Cardinale D, Sandri MT, Colombo A, Colombo N, Boeri M, Lamantia G, Civelli M, Peccatori F, Martinelli G, Fiorentini C, Cipolla CM (2004) Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation 109: 2749–2754
Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, Cinieri S, Martinelli G, Cipolla CM, Fiorentini C (2000) Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J Am Coll Cardiol 36: 517–522
Dodos F, Halbsguth T, Erdmann E, Hoppe UC (2008) Usefulness of myocardial performance index and biochemical markers for early detection of anthracycline-induced cardiotoxicity in adults. Clin Res Cardiol 97: 318–326
Feola M, Garrone O, Occelli M, Francini A, Biggi A, Visconti G, Albrile F, Bobbio M, Merlano M (2011) Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol 148: 194–198
Germanakis I, Kalmanti M, Parthenakis F, Nikitovic D, Stiakaki E, Vardas PE (2006) Correlation of plasma N-terminal pro-brain natriuretic peptide levels with left ventricle mass in children treated with anthracyclines. Int J Cardiol 108: 212–215
Gharib MI, Burnett AK (2002) Chemotherapy-induced cardiotoxicity: current practice and prospects of prophylaxis. Eur J Heart Fail 4: 235–242
Helmes M, Lim CC, Liao R, Bharti A, Cui L, Sawyer DB (2003) Titin determines the Frank-Starling relation in early diastole. J Gen Physiol 121: 97–110
Helmes M, Trombitas K, Granzier H (1996) Titin develops restoring force in rat cardiac myocytes. Circ Res 79: 619–626
Hequet O, Le QH, Moullet I, Pauli E, Salles G, Espinouse D, Dumontet C, Thieblemont C, Arnaud P, Antal D, Bouafia F, Coiffier B (2004) Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol 22: 1864–1871
Kilickap S, Barista I, Akgul E, Aytemir K, Aksoyek S, Aksoy S, Celik I, Kes S, Tekuzman G (2005) cTnT can be a useful marker for early detection of anthracycline cardiotoxicity. Ann Oncol 16: 798–804
Kouloubinis A, Kaklamanis L, Ziras N, Sofroniadou S, Makaritsis K, Adamopoulos S, Revela I, Athanasiou A, Mavroudis D, Georgoulias V (2007) ProANP and NT-proBNP levels to prospectively assess cardiac function in breast cancer patients treated with cardiotoxic chemotherapy. Int J Cardiol 122: 195–201
Latour-Pérez J, Coves-Orts FJ, Abad-Terrado C, Abraira V, Zamor J (2006) Accuracy of B-type natriuretic peptide levels in the diagnosis of left ventricular dysfunction and heart failure: a systematic review. Eur J Heart Fail 8: 390–399
Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, Orav EJ, Gelber RD, Colan SD (1995) Female sex and drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med 332: 1738–1743
Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, Colan SD, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Gelber RD, Sallan SE (2004) The effects of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med 351: 145–153
Mackay B, Ewer MS, Carrasco CH, Benjamin RS (1994) Assessment of anthracycline cardiomyopathy by endomyocardial biopsy. Ultrastruct Pathol 18: 203–211
Meinardi MT, Van Der Graaf WT, Gietema JA, Van Den Berg MP, Sleijfer DT, De Vries EG, Haaksma J, Boomsma F, Van Veldhuisen DJ (2002) Evaluation of long term cardiotoxicity after epirubicin containing adjuvant chemotherapy and locoregional radiotherapy for breast cancer using various detection techniques. Heart 88: 81–82
Meinardi MT, van Veldhuisen DJ, Gietema JA, Dolsma WV, Boomsma F, van den Berg MP, Volkers C, Haaksma J, de Vries EG, Sleijfer DT, van der Graaf WT (2001) Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J Clin Oncol 19: 2746–2753
Mele D, Pasanisi G, Capasso F, De Simone A, Morales MA, Poggio D, Capucci A, Tabacchi G, Sallusti L, Ferrari R (2006) Left intraventricular myocardial deformation dyssynchrony identifies responders to cardiac resynchronization therapy in patients with heart failure. Eur Heart J 27: 1070–1078
Mortensen SA, Olsen HS, Baandrup U (1986) Chronic anthracycline cardiotoxicity: haemodynamic and histopathological manifestations suggesting a restrictive endomyocardial disease. Br Heart J 55: 274–282
Pai VB, Nahata MC (2000) Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf 22: 263–302
Pichon MF, Cvitkovic F, Hacene K, Delaunay J, Lokiec F, Collignon MA, Pecking AP (2005) Drug-induced cardiotoxicity studied by longitudinal B-type natriuretic peptide assays and radionuclide ventriculography. In Vivo 19: 567–576
Romano S, Necozione S, Guarracini L, Fratini S, Cisternino P, di Orio F, Penco M (2009) Accuracy of N-terminal pro-brain natriuretic peptide in the identification of left ventricular dysfunction in high-risk asymptomatic patients. J Cardiovasc Med 10: 238–244
Rowan RA, Masek MA, Billingham ME (1988) Ultrastructural morphometric analysis of endomyocardial biopsies. Idiopathic dilated cardiomyopathy, anthracycline cardiotoxicity, and normal myocardium. Am J Cardiovasc Pathol 2: 137–144
Sandri MT, Salvatici M, Cardinale D, Zorzino L, Passerini R, Lentati P, Leon M, Civelli M, Martinelli G, Cipolla CM (2005) N-terminal pro-B-type natriuretic peptide after high-dose chemotherapy: a marker predictive of cardiac dysfunction? Clin Chem 51: 1405–1410
Sawaya H, Sebag IA, Plana JC, Januzzi JL, Ky B, Cohen V, Gosavi S, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer-Crosbie M (2011) Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol 107: 1375–1380
Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC (2010) Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis 53: 105–113
Schultz PN, Beck ML, Stava C, Vassilopoulou-Sellin R (2003) Health profiles in 5836 long-term cancer survivors. Int J Cancer 104: 488–495
Shan K, Lincoff AM, Young JB (1996) Anthracycline-induced cardiotoxicity. Ann Intern Med 125: 47–58
Singal PK, Iliskovic N (1998) Doxorubicin-induced cardiomyopathy. N Engl J Med 339: 900–905
Sparano JA, Brown DL, Wolff AC (2002) Predicting cancer therapy-induced cardiotoxicity: the role of troponins and other markers. Drug Saf 2: 301–311
Suzuki T, Hayashi D, Yamazaki T, Mizuno T, Kanda Y, Komuro I, Kurabayashi M, Yamaoki K, Mitani K, Hirai H, Nagai R, Yazaki Y (1998) Elevated B-type natriuretic peptide levels after anthracycline administration. Am Heart J 136: 362–363
Yeh ET, Bickford CL (2009) Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 53: 2231–2247
Wang SM, Greaser ML, Schultz E, Bulinski JC, Lin JJ, Lessard JL (1988) Studies on cardiac myofibrillogenesis with antibodies to titin, actin, tropomyosin, and myosin. J Cell Biol 107: 1075–1083
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Romano, S., Fratini, S., Ricevuto, E. et al. Serial measurements of NT-proBNP are predictive of not-high-dose anthracycline cardiotoxicity in breast cancer patients. Br J Cancer 105, 1663–1668 (2011). https://doi.org/10.1038/bjc.2011.439
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2011.439
Keywords
This article is cited by
-
Electrocardiographic and biochemical analysis of anthracycline induced cardiotoxicity in breast cancer patients from Southern Sri Lanka
BMC Cancer (2023)
-
Coronary artery disease, left ventricular function and cardiac biomarkers determine all-cause mortality in cancer patients—a large monocenter cohort study
Clinical Research in Cardiology (2023)
-
The prognostic value of global myocardium strain by CMR-feature tracking in immune checkpoint inhibitor–associated myocarditis
European Radiology (2022)
-
Circulating Biomarkers for Cardiotoxicity Risk Prediction
Current Treatment Options in Oncology (2021)
-
Trastuzumab-induced cardiotoxicity: a review of clinical risk factors, pharmacologic prevention, and cardiotoxicity of other HER2-directed therapies
Breast Cancer Research and Treatment (2021)