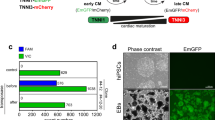
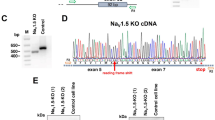
Abstract
Individuals with congenital or acquired prolongation of the QT interval, or long QT syndrome (LQTS), are at risk of life-threatening ventricular arrhythmia1,2. LQTS is commonly genetic in origin but can also be caused or exacerbated by environmental factors1,3. A missense mutation in the L-type calcium channel CaV1.2 leads to LQTS in patients with Timothy syndrome4,5. To explore the effect of the Timothy syndrome mutation on the electrical activity and contraction of human cardiomyocytes, we reprogrammed human skin cells from Timothy syndrome patients to generate induced pluripotent stem cells, and differentiated these cells into cardiomyocytes. Electrophysiological recording and calcium (Ca2+) imaging studies of these cells revealed irregular contraction, excess Ca2+ influx, prolonged action potentials, irregular electrical activity and abnormal calcium transients in ventricular-like cells. We found that roscovitine, a compound that increases the voltage-dependent inactivation of CaV1.2 (refs 6–8), restored the electrical and Ca2+ signalling properties of cardiomyocytes from Timothy syndrome patients. This study provides new opportunities for studying the molecular and cellular mechanisms of cardiac arrhythmias in humans, and provides a robust assay for developing new drugs to treat these diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Keating, M. T. The long QT syndrome. A review of recent molecular genetic and physiologic discoveries. Medicine 75, 1–5 (1996)
Huikuri, H. V., Castellanos, A. & Myerburg, R. J. Sudden death due to cardiac arrhythmias. N. Engl. J. Med. 345, 1473–1482 (2001)
Paakkari, I. Cardiotoxicity of new antihistamines and cisapride. Toxicol. Lett. 127, 279–284 (2002)
Splawski, I. et al. CaV1.2 Ca2+ channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell 119, 19–31 (2004)
Splawski, I. et al. Severe arrhythmia disorder caused by cardiac L-type Ca2+ channel mutations. Proc. Natl Acad. Sci. USA 102, 8089–8096 (2005)
Yarotskyy, V. & Elmslie, K. S. Roscovitine, a cyclin-dependent kinase inhibitor, affects several gating mechanisms to inhibit cardiac L-type (CaV1.2) Ca2+ channels. Br. J. Pharmacol. 152, 386–395 (2007)
Yarotskyy, V., Gao, G., Peterson, B. Z. & Elmslie, K. S. The Timothy syndrome mutation of cardiac CaV1.2 (L-type) channels: multiple altered gating mechanisms and pharmacological restoration of inactivation. J. Physiol. (Lond.) 587, 551–565 (2009)
Yarotskyy, V. et al. Roscovitine binds to novel L-channel (CaV1.2) sites that separately affect activation and inactivation. J. Biol. Chem. 285, 43–53 (2010)
Roden, D. M. & Viswanathan, P. C. Genetics of acquired long QT syndrome. J. Clin. Invest. 115, 2025–2032 (2005)
Chen, L. et al. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc. Natl Acad. Sci. USA 104, 20990–20995 (2007)
Roden, D. M. Clinical practice. Long-QT syndrome. N. Engl. J. Med. 358, 169–176 (2008)
Moretti, A. et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 363, 1397–1409 (2010)
Reuter, H. Ion channels in cardiac cell membranes. Annu. Rev. Physiol. 46, 473–484 (1984)
Flucher, B. E. & Franzini-Armstrong, C. Formation of junctions involved in excitation-contraction coupling in skeletal and cardiac muscle. Proc. Natl Acad. Sci. USA 93, 8101–8106 (1996)
Seisenberger, C. et al. Functional embryonic cardiomyocytes after disruption of the L-type α1C (Cav1.2) Ca2+ channel gene in the mouse. J. Biol. Chem. 275, 39193–39199 (2000)
Takeshima, H. et al. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. EMBO J. 17, 3309–3316 (1998)
Yazawa, M. et al. TRIC channels are essential for Ca2+ handling in intracellular stores. Nature 448, 78–82 (2007)
Barrett, C. F. & Tsien, R. W. The Timothy syndrome mutation differentially affects voltage- and calcium-dependent inactivation of CaV1.2 L-type Ca2+ channels. Proc. Natl Acad. Sci. USA 105, 2157–2162 (2008)
Thiel, W. H. et al. Proarrhythmic defects in Timothy syndrome require calmodulin kinase II. Circulation 118, 2225–2234 (2008)
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007)
Yu, J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007)
Aoi, T. et al. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321, 699–702 (2008)
He, J. Q., Ma, Y., Lee, Y., Thomson, J. A. & Kamp, T. J. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ. Res. 93, 32–39 (2003)
Zhang, J. et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 104, e30–e41 (2009)
Brunner, M. et al. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J. Clin. Invest. 118, 2246–2259 (2008)
Meijer, L. et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243, 527–536 (1997)
Tran, T. H. et al. Wnt3a-induced mesoderm formation and cardiomyogenesis in human embryonic stem cells. Stem Cells 27, 1869–1878 (2009)
Acknowledgements
We thank K. Timothy and the Timothy syndrome patients who participated in this study; U. Francke for discussion and for providing karyotyping expertise; A. Cherry and D. Bangs for fibroblast isolation; K. C. Chan for iPSC cultures; O. Shcheglovitov for help with electrophysiological recordings; and A. Olson for help with the confocal microscope. Funding was provided by grants from the Japan Society for the Promotion for Science and the American Heart Association Western States to M.Y., and a National Institutes of Health Director’s Pioneer Award, a grant from the Simons Foundation to R.E.D and gifts from L. Miller, B. and F. Horowitz and M. McCaffery.
Author information
Authors and Affiliations
Contributions
M.Y. and R.E.D. designed research and wrote the manuscript; J.A.B., J.H. and R.E.D recruited the Timothy syndrome patients; M.Y. and X.J. generated and characterized control and Timothy syndrome iPSCs; A.M.P. conducted karyotyping; M.Y. performed generation and characterization of human cardiomyocytes, whole-cell patch clamp, and Ca2+ imaging; M.Y. and B.H. analysed cardiomyocytes contraction rates.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-8 with legends and Supplementary Tables 1-2. (PDF 1874 kb)
Supplementary Methods
This file contains Supplementary Materials and Methods. (PDF 149 kb)
Supplementary Movie 1
This movie shows control EBs (round) spontaneously contracting at d37. (MOV 9615 kb)
Supplementary Movie 2
This movie shows control EBs (flat) spontaneously contracting at d37. (MOV 9497 kb)
Supplementary Movie 3
This movie shows TS EBs (round) spontaneously contracting at d37. (MOV 10200 kb)
Supplementary Movie 4
This movie shows TS EBs (flat) spontaneously contracting at d37. (MOV 9497 kb)
Rights and permissions
About this article
Cite this article
Yazawa, M., Hsueh, B., Jia, X. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011). https://doi.org/10.1038/nature09855
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09855
This article is cited by
-
Modeling human neurodevelopmental diseases with brain organoids
Cell Regeneration (2022)
-
Increased CaV1.2 late current by a CACNA1C p.R412M variant causes an atypical Timothy syndrome without syndactyly
Scientific Reports (2022)
-
Sigma non-opioid receptor 1 is a potential therapeutic target for long QT syndrome
Nature Cardiovascular Research (2022)
-
Increased cytosolic calcium buffering contributes to a cellular arrhythmogenic substrate in iPSC-cardiomyocytes from patients with dilated cardiomyopathy
Basic Research in Cardiology (2022)
-
“Cutting the Mustard” with Induced Pluripotent Stem Cells: An Overview and Applications in Healthcare Paradigm
Stem Cell Reviews and Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.