Article Text
Abstract
Background Studies have suggested that women’s reproductive factors are associated with the risk of cardiovascular disease (CVD); however, findings are mixed. We assessed the relationship between reproductive factors and incident CVD in the UK Biobank.
Methods Between 2006 and 2010, the UK Biobank recruited over 500 000 participants aged 40–69 years across the UK. During 7 years of follow-up, 9054 incident cases of CVD (34% women), 5782 cases of coronary heart disease (CHD) (28% women), and 3489 cases of stroke (43% women) were recorded among 267 440 women and 215 088 men without a history of CVD at baseline. Cox regression models yielded adjusted hazard ratios (HRs) for CVD, CHD and stroke associated with reproductive factors.
Results Adjusted HRs (95% CI) for CVD were 1.10 (1.01 to 1.30) for early menarche (<12 years), 0.97 (0.96 to 0.98) for each year increase in age at first birth, 1.04 (1.00 to 1.09) for each miscarriage, 1.14 (1.02 to 1.28) for each stillbirth, and 1.33 (1.19 to 1.49) for early menopause (<47 years). Hysterectomy without oophorectomy or with previous oophorectomy had adjusted HRs of 1.16 (1.06 to 1.28) and 2.30 (1.20 to 4.43) for CVD. Each additional child was associated with a HR for CVD of 1.03 (1.00 to 1.06) in women and 1.03 (1.02 to 1.05) in men.
Conclusions Early menarche, early menopause, earlier age at first birth, and a history of miscarriage, stillbirth or hysterectomy were each independently associated with a higher risk of CVD in later life. The relationship between the number of children and incident CVD was similar for men and women.
- cardiovascular disease
- women
- menarche
- parity
- pregnancy complications
- hysterectomy
- oophorectomy
- menopause
Statistics from Altmetric.com
Introduction
Cardiovascular disease (CVD) remains the leading cause of death and disability worldwide in both sexes.1 Most of the burden of CVD can be explained by a set of traditional risk factors that affect both men and women, including elevated blood pressure, smoking, overweight and obesity, diabetes mellitus, and elevated cholesterol. In women, there is increasing evidence to suggest that several reproductive factors may be associated with the risk of CVD in later life.2–4 However, the findings are mixed and inconsistent, which, in addition to methodological limitations of previous studies, may be attributed to differences in women’s reproductive patterns between populations and over time.
Most, but not all, studies have reported that an early menarche and an early menopause are associated with an increased risk of CVD in later life.2 3 5–7 A history of miscarriage or recurrent miscarriage has been linked to an increased risk of coronary heart disease (CHD), with weaker evidence for stroke.8 Few studies have examined the association between stillbirth and the risk of CVD, with inconsistent findings.2 9–11 Moreover, while several studies have found that parity (number of children) is associated with the risk of maternal CVD, recent reports found that the association between the number of children and the risk of CVD was similar between men and women, suggesting that the association between high parity and CVD in later life may be largely explained by social, cultural, psychological and behavioural factors associated with parenthood and childrearing.12–14 Studies have shown that hysterectomy might be associated with an increased risk for CVD, but the evidence is inconclusive and may differ between women with and without oophorectomy.15–17
In a previous cross-sectional analysis in the UK Biobank, we demonstrated that several reproductive factors were associated with levels of general and central body adiposity.18 In the present study, we use prospective data from 482 000 women and men in the UK Biobank to assess the relationship between reproductive factors and the risk of incident CVD in later life.
Methods
Study population
The UK Biobank is a large prospective, population-based cohort study.19 Between 2006 and 2010, over 500 000 women and men (5.5% response rate) aged 40–69 years at baseline attended one of the 22 centres across the UK. Participants provided electronic informed consent and completed questionnaires on their lifestyle, environment and medical history, had physical and functional measures performed, and had samples of blood, urine and saliva collected. We included 267 440 women and 215 088 men without a history of CVD at baseline.
Reproductive factors
Self-reported reproductive factors included in this analysis were age at menarche, number of live births (henceforth, children), age at first birth, history and number of miscarriage(s) and stillbirth(s), history of, and age at hysterectomy and oophorectomy, menopausal status and, if menopausal, age at natural menopause. Men were asked how many children they had fathered. Early menarche was defined as age at first menstrual period before the age of 12 years. Early menopause was defined as the permanent absence of menstrual periods before the age of 47 years.
Study endpoints
The main study endpoints were the incidence of fatal or non-fatal myocardial infarction (MI) (henceforth CHD) or stroke, as defined by the UK Biobank. Outcome adjudication involved linkage with hospital admissions data in England, Scotland and Wales and national death register data to identify the date of the first known CHD or stroke after the date of baseline assessment. Incident CHD was defined by codes I21, I22, I23, I24.1 or I25.2 in the 10th edition of the International Classification of Diseases (ICD-10). Incident stroke was defined by ICD-10 codes I60, I61, I63, or I64. Follow-up started at inclusion in the UK Biobank study and ended on 1 March 2016, or on the first fatal or non-fatal CHD or stroke, for all participants.CVD was defined as the first occurrence of either CHD or stroke.
Statistical analyses
Baseline characteristics are presented as means (SD) for continuous variables and as percentages for categorical variables. Cox proportional hazards models were used to estimate hazard ratios and 95% confidence intervals (HR, 95% CI) for the study endpoints associated with reproductive factors. The Cox proportional hazards assumption was checked using log cumulative hazard plots and appeared to be reasonable. Penalised smoothing splines were used to graphically examine the shape of associations between reproductive factors and the study endpoints. Linear trends were reported where the associations were approximately log-linear. Categorical analyses were conducted for all reproductive factors. For comparisons involving more than two groups, confidence intervals were estimated using floating absolute risks. Models were adjusted for age at study entry, socioeconomic status (measured by the Townsend deprivation index), smoking status, systolic blood pressure, body mass index (BMI) and history of diabetes mellitus. Subgroup analyses were conducted by age (≤60 years vs >60 years), socioeconomic status (Townsend deprivation index at or below vs above the English median), smoking status (never vs ever), and BMI (≤25 kg/m2 vs >25 kg/m2). P values for interaction were obtained by adding an interaction term to the models. R version 3.1.2 was used for all analyses.
Results
For women, the mean age at baseline was 56 years, 51% were from the most socioeconomically advantaged third of the UK population and 60% had never smoked (table 1). The mean age at menarche was 13 years, 85% had been pregnant and 44% of women had given birth to two children. The mean age at first birth was 26 years. Twenty-five per cent of women had a history of miscarriage and 3% had had a stillbirth. Sixty-one per cent of women were postmenopausal, with a mean age at natural menopause of 50 years. Forty-two per cent of men had fathered two children. During a median of 7.1 years (Q1: 6.4; Q3: 7.8) follow-up, 9054 incident cases of CVD were recorded (34% women), including 5782 cases of CHD (28% women) and 3489 cases of stroke (43% women).
Baseline characteristics of study participants
Age at menarche
Women who had their menarche before the age of 12 years were at a higher risk of CVD than those who had their menarche at an older age (figure 1 and online supplementary etables 1). The age-adjusted HR (95% CI) of CVD associated with an early menarche (<12 years) was 1.18 (1.09 to 1.29), which attenuated to 1.10 (1.01 to 1.30) following further adjustment, without evidence for differences between subpopulations (online supplementary etables 1 and 2). The age-adjusted and multiple-adjusted HRs, respectively, associated with an early menarche were 1.16 (1.03 to 1.30) and 1.05 (0.93 to 1.18) for CHD and 1.22 (1.08 to 1.37) and 1.17 (1.03 to 1.32) for stroke, with minimal differences across subpopulations (online supplementary etables 1, 3 and 4). The risk of CHD associated with an early menarche differed by age group; the adjusted HR was 0.82 (0.67 to 1.01) for those ≤60 years and 1.21 (1.04 to 1.41) for those aged > 60 years (P=0.01) (online supplementary etables 3). Women with a later age at menarche did not have a higher risk of CVD, CHD or stroke compared with those who had their menarche at 13 years of age.
Supplementary file 1
Penalised spline plots with adjusted hazard ratios (95% CI) for cardiovascular disease associated with women’s age at menarche and age at natural menopause. Analyses are adjusted for age, Townsend deprivation index, smoking status, systolic blood pressure, history of diabetes and body mass index.
Number of children
Compared with women and men without children, those with children were at a significantly higher risk of CHD, but not of CVD or stroke; the multiple-adjusted HRs (95% CI) were 1.21 (1.05 to 1.40) in women and 1.13 (1.04 to 1.23) in men for CHD and 0.97 (0.84 to 1.11) in women and 0.99 (0.88 to 1.11) in men for stroke (table 2 and online supplementary etables 5 and 6). The relationship between the number of children and the risk of CVD, CHD and stroke was similar between the sexes (figure 2). The adjusted HR for CVD associated with each child was 1.03 (1.00 to 1.06) in women and 1.03 (1.02 to 1.05) in men. Corresponding HRs for CHD and stroke were 1.06 (1.02 to 1.10) and 1.01 (0.96 to 1.05) in women and 1.04 (1.02 to 1.06) and 1.01 (0.98 to 1.05) in men. There was some indication that the risk of CVD associated with the number of children in women was stronger among those with a higher socioeconomic status and ever smokers (P=0.04) (online supplementary etables 2).
Adjusted hazard ratios (HR, 95% CI) for coronary heart disease (CHD) and stroke associated with the number of children in women and men. Analyses are adjusted for age, Townsend deprivation index, smoking status, systolic blood pressure, history of diabetes and body mass index. The HRs are plotted on a floating absolute scale. The area of each square is inversely proportional to the SE of the log HR. Vertical lines indicate the corresponding 95% CI.
Adjusted hazard ratios (HR, 95% CI) for CVD, CHD and stroke associated with women’s reproductive factors
Age at first birth
There was an inverse relationship between age at first birth and the risk of CVD, CHD and stroke, which attenuated marginally following adjustment for socioeconomic status and lifestyle factors (figure 3, table 2 and online supplementary etables 5). The adjusted HRs (95% CI) for every year increase in age at first birth were 0.97 (0.96 to 0.98) for CVD, 0.96 (0.95 to 0.97) for CHD, and 0.98 (0.97 to 0.99) for stroke, with little heterogeneity across population subgroups (online supplementary etables 2–4).
Adjusted hazard ratios (HR, 95% CI) for coronary heart disease (CHD) and stroke associated with age at first live birth. Analyses are adjusted for age, Townsend deprivation index, smoking status, systolic blood pressure, history of diabetes and body mass index. The HRs are plotted on a floating absolute scale. The area of each square is inversely proportional to the SE of the log HR. Vertical lines indicate the corresponding 95% CI.
Miscarriage and stillbirth
A history of miscarriage was associated with a higher risk of CHD, but not of CVD or stroke; multiple-adjusted HRs (95% CI) were 1.07 (0.98 to 1.17) for CVD, 1.14 (1.01 to 1.29) for CHD, and 0.98 (0.86 to 1.12) for stroke (table 2). The adjusted HRs associated with each miscarriage were 1.04 (1.00 to 1.09) for CVD, 1.06 (1.00 to 1.13) for CHD, and 1.01 (0.95 to 1.09) for stroke. A history of stillbirth was associated with a higher risk of CVD and stroke, but not of CHD; adjusted HRs were 1.22 (1.01 to 1.46) for CVD, 0.98 (0.74 to 1.28) for CHD, and 1.44 (1.12 to 1.85) for stroke (table 2). The adjusted HRs associated with each additional stillbirth were 1.14 (1.02 to 1.28) for CVD, 1.03 (0.85 to 1.24) for CHD, and 1.21 (1.05 to 1.40) for stroke. The results were broadly consistent across population subgroups (online supplementary etables 2–4). However, there was some indication that the risks of CVD and CHD associated with a history of miscarriage were stronger among women with a higher socioeconomic status (P=0.03) (online supplementary etables 2–3).
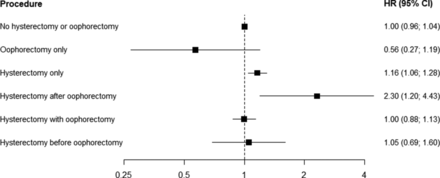
Hysterectomy and oophorectomy
A history of oophorectomy was not associated with the risk of CVD, CHD or stroke (table 2 and online supplementary etables 5). A history of hysterectomy was associated with the risk of CVD and CHD, but not of stroke; multiple-adjusted HRs (95% CI) were 1.12 (1.03 to 1.22), 1.20 (1.07 to 1.34), and 1.05 (0.93 to 1.19), respectively. There was no evidence for differences between subpopulations (online supplementary etables 2–4). Age at oophorectomy and age at hysterectomy were each inversely associated with the risk of CVD, CHD and stroke; adjusted HRs for CVD were 0.97 (0.95 to 0.98) for a 1 year increase in age at oophorectomy and 0.98 (0.97 to 0.99) for a 1 year increase in age at hysterectomy (table 2). Moreover, the increased risk of CVD and CHD, but not of stroke, associated with a history of hysterectomy differed by history and timing of oophorectomy, with those who had an oophorectomy before hysterectomy at the highest risk (figure 4 and online supplementary etables 7).
Adjusted hazard ratios (HR, 95% CI) for cardiovascular disease associated with history and timing of hysterectomy and oophorectomy. Analyses are adjusted for age, Townsend deprivation index, smoking status, systolic blood pressure, history of diabetes and body mass index. The HRs are plotted on a floating absolute scale. Horizontal lines indicate the corresponding 95% CI.
Age at natural menopause
An early age at natural menopause was associated with a greater risk of CVD, CHD and stroke (figure 1 and online supplementary etables 1). The age-adjusted HRs (95% CI) were 1.44 (1.29 to 1.61) for CVD, 1.43 (1.22 to 1.67) for CHD, and 1.50 (1.28 to 1.75) for stroke; these attenuated to 1.33 (1.19 to 1.49), 1.29 (1.10 to 1.51), and 1.42 (1.21 to 1.66), respectively, following adjustment for socioeconomic status and lifestyle factors. There was no evidence that the associations differed across subpopulations (online supplementary etables 2–4).
Discussion
In this large prospective study of 482 000 women and men in the UK Biobank, several common reproductive factors were associated with the risk of CVD in later life. Among women, early menarche, early menopause, age at first birth, and a history of miscarriage, stillbirth or hysterectomy were each associated with a higher risk of CVD in later life. The relationship between the number of children and the risk of CVD was similar for men and women. While the strengths of the relationships differed by CVD subtype, the results were broadly consistent across major subpopulations and were generally robust to adjustment for several socioeconomic and biological confounders and mediators.
Our results are in agreement with some, but not all, previous studies that reported that early menarche was associated with an increased risk of CVD.2 3 6 7 The most comprehensive report to date on the association between menarche and CVD risk came from the UK Million Women Study, which found a U-shaped relationship between age at menarche with CVD risk, with both early (≤10 years) and late (≥17 years) menarche being associated with an increased risk of CHD and stroke.6 The mechanisms through which age at menarche is linked to CVD risk in later life are uncertain. Menarche is a marker of timing of puberty and early menarche has been linked to childhood obesity.20 21 Several studies, including ours, have shown that an earlier menarche was associated with high levels of adiposity in adulthood.18 22 However, the findings of the present study were robust to adjustment for BMI and were similar between healthy weight and overweight or obese women, suggesting that other mechanisms are involved. Genome-wide association studies have reported that age at menarche is closely linked to several pathways, including the regulation of energy homeostasis, body weight and hormonal levels.23 Mendelian randomisation analyses will help to examine whether the link between early menarche and CVD risk is causal and which mechanisms are involved.
The present study demonstrated that the association between the number of children and the risk of CHD and stroke was largely identical between women and men. These findings agree with our recent study of 500 000 men and women from the China Kadoorie Biobank, which also showed a similar increased risk of incident CHD and stroke, as well as incident diabetes, with an increasing number of children in both sexes.13 14 Hence, the link between repeated childbirth and CVD risk seen in women may be more likely to be explained by social, cultural, psychological and behavioural factors related to parenthood and childbearing than by biological factors related to pregnancy and childbearing. Previous studies have reported that an early age at first birth was associated with unfavourable cardiovascular risk profiles in both men and women.24 25 Thus, it is conceivable that the inverse relationship between age at first birth and the risk of later CHD and stroke in women reported here is also predominantly explained by factors other than reproductive ones.
Miscarriage and stillbirth may be aetiologically linked to CVD by shared underlying vascular pathology, including systemic inflammation and endothelial dysfunction, that could not only result in placental dysfunction and pregnancy loss, but may also be implicated in the risk of CVD.26–28 A previous meta-analysis reported that a history of miscarriage and recurrent miscarriage were associated with a higher risk of CHD, but not stroke.8 A study among 60 000 women in Scotland reported that repeated miscarriage was associated with an increased risk of CHD, but not stroke.29 In contrast, a study of over 1 million women in Denmark found graded relationships between the number of miscarriages and the risks of both MI and stroke.11 A history of stillbirth has also been associated with increased risks of CVD.10 11 This study is the first to suggest that miscarriage is more strongly associated with CHD whereas stillbirth is more strongly associated with stroke, heightening the need for specific research into the pathways through which pregnancy loss is implicated in the pathophysiology of vascular disease subtypes.
In agreement with previous studies,5 we observed that an early age at natural menopause was associated with a higher risk of both CHD and stroke. The higher risk of CVD among women with an early menopause has commonly been ascribed to the adverse effects of loss of ovarian function and a subsequent deficiency of endogenous oestrogens on a woman’s cardiovascular risk profile. However, it has also been suggested that it is not menopause that adversely affects cardiovascular risk but rather that cardiovascular risk factors determine the age at menopause, possibly through direct effects on the endocrine system or by inducing ischaemic damage in the ovaries.30 Unlike previous studies,5 oophorectomy was not associated with an increased risk of CVD in this study. However, age at oophorectomy was inversely associated with the risk of CVD in later life, suggesting that the timing of surgical removal of the ovaries and subsequent abrupt transition into the menopause, rather than the procedure itself, might underpin the previously reported findings. A history of hysterectomy was associated with a higher risk of CHD, but not of stroke, with some evidence that the higher risk was confined to women without concomitant oophorectomy or with a previous oophorectomy. In contrast, previous studies have suggested that oophorectomy at the time of hysterectomy added further to the risk of CVD in pre-menopausal women,15–17 which could be explained by a more adverse initial risk profile or premature ovarian failure and hormone-related effects on serum lipids and subclinical atherosclerosis.
The strengths of this study include the large sample size, prospective design and availability of detailed information on reproductive and other lifestyle factors. Information on the number of children and men enabled us to assess the association between parity and CVD simultaneously in women and men. This study also has some limitations. First, information on reproductive factors was self-reported and solicited several years after the reproductive events occurred. This could have led to measurement error, which, if at random, would have underestimated the true strengths of the observed associations. Second, although our findings were robust to adjustment for several major confounders, the observed associations may have been subject to unmeasured or residual confounding, particularly those related to physiological, cultural or socioeconomic factors early in life and before or during pregnancy. Our results may also have been liable to reverse causality as certain conditions, such as childhood obesity or type 1 diabetes, may determine a woman’s reproductive life course and may also confer a higher risk of cardiovascular conditions in later life. Furthermore, despite the large sample size, the number of events was limited in some of the analyses. Finally, no data were available on several pregnancy-related factors that have been shown to be associated with the risk of CVD in later life, including breastfeeding, gestational diabetes, gestational obesity, preeclampsia and polycystic ovary syndrome.
In conclusion, this large prospective study among 482 000 women and men in the UK Biobank demonstrated that several reproductive factors are associated with the risk of CVD in later life. Future studies are needed to confirm the present findings and to clarify the biological, behavioural and social mechanisms involved.
Key messages
What is already known about this subject?
It is often hypothesised that certain factors relating to reproduction and pregnancy may have an adverse effect on a woman’s future risk of cardiovascular disease (CVD). Findings in previous studies have, however, been mixed, leading to an inconclusive evidence base.
What does this study add?
This large prospective study found that early menarche, early menopause, earlier age at first birth, and a history of miscarriage, stillbirth or hysterectomy, were each associated with a higher risk of CVD in later life, after controlling for key cardiovascular risk factors. Women who had given birth to more children also had higher subsequent CVD risk, but this is unlikely to have been due to a biological cause, as some have hypothesised, because a similar effect was found when analysing the number of children fathered by men.
How might this impact on clinical practice?
More frequent cardiovascular screening would seem to be sensible among women who are early in their reproductive cycle, or who have a history of adverse reproductive events or a hysterectomy, as this might help to delay or prevent their onset of CVD. Cardiovascular risk stratification that accounts for key reproductive factors should be considered to help address the considerable CVD risk among women.
Acknowledgments
This research has been conducted using the UK Biobank Resource. Permission to use the UK Biobank Resource was approved by the Access Sub-Committee of the UK Biobank Board.
References
Supplementary materials
Press release
Files in this Data Supplement:
Footnotes
Contributors SP and MW conceived the study, interpreted the data, and approved the final version of the manuscript. SP conducted the statistical analyses and prepared the first draft of the manuscript, which was critically revised by MW.
Funding SP is supported by a UK Medical Research Council Skills Development Fellowship (MR/P014550/1). MW is supported by a NHMRC Fellowship (APP108026).
Competing interests None declared.
Patient consent Obtained.
Ethics approval UK Biobank has obtained Research Tissue Bank approval from its governing Research Ethics Committee, as recommended by the National Research Ethics Service. No separate ethics approval was required. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Researchers can apply to use the UK Biobank resource and access the data used. No additional data are available.