Article Text
Statistics from Altmetric.com
In patients with severe mitral regurgitation who meet criteria for operative intervention, the preferred surgical approach is mitral valve repair, rather than replacement. Mitral valve repair avoids the disadvantages of a prosthetic valve, including suboptimal hemodynamics, long-term warfarin anticoagulation (with a mechanical valve), limited valve durability (with a bioprosthetic valve) and an increased risk of thromboembolic events, endocarditis, and paravalvular regurgitation with both types of prosthetic valve. In addition, patients undergoing mitral valve repair have a lower operative mortality (<1%), better post-operative left ventricular systolic function and improved long-term outcomes compared to patients undergoing mitral valve replacement. After successful mitral valve repair, over 80% of patients remain free of significant mitral regurgitation 15 to 20 years later.
However, the likelihood of a successful mitral valve repair varies widely between medical centers reflecting surgical team expertise and experience. Moreover, individual variation in mitral valve anatomy determines whether valve repair is possible and what specific surgical techniques are required in each patient. Echocardiographic evaluation both before and during operative repair is routine and three-dimensional (3D) imaging has greatly increased our appreciation of valve anatomy. In a milestone paper in this issue of Heart, Dr Mantovani and colleagues (see page 1111) convincingly demonstrate that 3D echocardiography also provides unique information that can help guide the surgical approach. In a series of 49 patients undergoing mitral valve repair, about 1/3 had a cleft-like indentation (CLI) in the posterior mitral leaflet, all of which required surgical closure to ensure valve competency (figure 1). The online video abstract http://heart.bmj.com/content/early/2015/05/20/heartjnl-2014-307016.full nicely demonstrates these mitral valve clefts, which clearly have implications for transcatheter based mitral valve intervention, as well as for surgical repair.
Example of a patient with myxomatous mitral valve disease (MMVD) and CLI with recording of 3D imaging and operative view imaging. Left image: 3D transoesophageal echocardiographic view of the mitral valve from the left atrial position. Right image: direct mitral valve view during surgical inspection. The red arrow indicates the CLI of the posterior mitral valve leaflet; the yellow arrow head indicates the prolapsing scallop (middle) of the posterior leaflet. Note, the visible deep indentation between P2 and P3 by both 3D imaging and direct viewing of the mitral valve. CLI, cleft-like indentation.
In the accompanying editorial, Dr McCarthy (see page 1087) comments “Surgeons, of course, have the ultimate real-time 3D imaging using their magnifying loupes and have noted the indentations over the decades” but there has been little previous discussion of whether CLI should be closed (figure 2). He further suggests that “if [CLI closure] were routinely performed in surgical centres, it likely would lead to less residual mitral regurgitation after mitral valve repair, which is particularly important considering that many surgeons do not repair mitral regurgitation regularly”.
After (A) resection and (B) P2 reconstruction, (C) the indentation (or cleft-like indentation) may be more prominent and (D) are easily closed to avoid residual MR.
In patients with myocardial infarction (MI), those with concurrent chronic obstructive pulmonary disease (COPD) have a higher mortality compared to patients without COPD. Based on the UK Myocardial Ischemia National Audit Project (MINAP) database of over 300 thousand patients with a first MI, Mr Rothnie and colleagues (see page 1103) used logistic regression to compare in-hospital (4.6% vs 3.2%) and 180 day (12.8% vs. 7.7%) mortality between those with and without COPD to determine what factors might explain this mortality difference. These data show that adjustment for in-hospital factors substantially reduced the effect of COPD on in-hospital mortality. Similarly, adjusting for in-hospital factors and secondary prevention reduced the effect of COPD on 180-day mortality. Thus, they conclude that higher mortality in COPD patients with an MI is largely explained by delays in diagnosis, decreased use of reperfusion therapy or angiography, and suboptimal secondary prevention medications.
We need to “mind and mend the gap” according to Drs Rajagopalan and Brook (see page 1085) who note: “this ‘mortality gap’ in patients with COPD presenting with MI may be ‘mended’ to a substantive degree through scrupulous attention to hospital care process and the fastidious institution of appropriate secondary prevention therapies. On the other hand, the outcome ‘gap’ was lessened but not eliminated. This strongly supports that other factors including biological susceptibility also underlie the relationship between COPD and residual risk for mortality. Given the rising global burden of COPD and the persistent associations with excess cardiovascular events, there are compelling reasons to consider targeting COPD-specific disease pathways causally related to a potentiated cardiovascular disease process” (figure 3).
Inter-relationships between COPD and heightened cardiovascular risk. CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction.
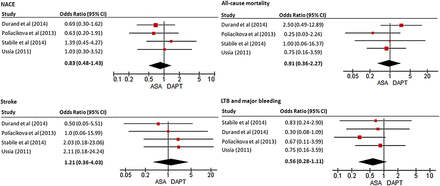
If you, like me, wonder about the optimal anti-platelet therapy after transcatheter aortic valve implantation (TAVI), the study by Dr Hassell and colleagues (see page 1118) in this issue of Heart provides some welcome objective data to guide our clinical decisions. These authors performed a systematic review with pooled analysis of individual patient data comparing dual antiplatelet therapy (DAPT) to aspirin alone (ASA) with a primary composite endpoint of adverse clinical and cerebral events at 1 month. The number of adverse events (defined as all-cause mortality, acute coronary syndrome, stroke, and major bleeding) did not differ between patients receiving DAPT (15%) or ASA alone (13%) but there was a trend towards less serious bleeding in the ASA only group (figure 4).
Relationship of antiplatelet therapy (aspirin vs dual antiplatelet therapy (DAPT)) with outcomes in individual studies. Forest plot for net adverse clinical and cerebral events (NACEs), all-cause mortality, stroke and life-threatening bleeding as outcomes. Log OR, values >1.0 indicate in favour of DAPT. The ORs with its 95% CI for each study are denoted by red squares and black lines. The size of the red square is proportional to the weight assigned to the study in the pooled estimate. The combined OR estimate for each endpoint is represented by a black diamond, where diamond width corresponds to 95% CI bounds. ASA, aspirin; LTB, life-threatening bleeding.
In an editorial, Dr Iung (see page 1089) reminds us that both the current European and American guidelines recommend DAPT early after TAVI, followed by long-term ASA alone. He concludes “The analysis by Hassell et al does not provide enough evidence to change existing recommendations. However, they have the considerable merit to challenge them and draw attention to the need to optimise each step of the management of patients undergoing TAVI. Early and late antithrombotic therapies used after TAVI presently rely on low levels of evidence. Randomised trials are ongoing and much needed to provide evidence-based answers to the questions on the respective roles of antiplatelet and anticoagulant therapies in these particular patients who are at high risk for both thromboembolism and bleeding”.
The Education in Heart article in this issue summarizes the diagnosis and treatment of pericarditis by Prof. Imazio (see page 1159). This concise and nicely illustrated review will be of value to all clinical cardiologists. The list of “red flags” and the flow diagrams for diagnosis are particularly helpful, along with a clear presentation of the therapeutic options.
The Image Challenge case (see page 1110) asks you to review an intracoronary optical coherence tomographic image to make the clinical diagnosis in a patient with known coronary disease who presented with acute chest pain and ECG changes. Even if this is not a modality you use yourself, the question and discussion will increase your knowledge base.
Linked Articles
- Editorial
- Editorial
- Valvular heart disease
- Education in Heart
- Valvular heart disease
- Editorial
- Image challenge
- Special populations