Article Text
Abstract
Objective To examine the association between chocolate intake and the risk of future cardiovascular events.
Methods We conducted a prospective study using data from the European Prospective Investigation into Cancer (EPIC)-Norfolk cohort. Habitual chocolate intake was quantified using the baseline food frequency questionnaire (1993–1997) and cardiovascular end points were ascertained up to March 2008. A systematic review was performed to evaluate chocolate consumption and cardiovascular outcomes.
Results A total of 20 951 men and women were included in EPIC-Norfolk analysis (mean follow-up 11.3±2.8 years, median 11.9 years). The percentage of participants with coronary heart disease (CHD) in the highest and lowest quintile of chocolate consumption was 9.7% and 13.8%, and the respective rates for stroke were 3.1% and 5.4%. The multivariate-adjusted HR for CHD was 0.88 (95% CI 0.77 to 1.01) for those in the top quintile of chocolate consumption (16–99 g/day) versus non-consumers of chocolate intake. The corresponding HR for stroke and cardiovascular disease (cardiovascular disease defined by the sum of CHD and stroke) were 0.77 (95% CI 0.62 to 0.97) and 0.86 (95% CI 0.76 to 0.97). The propensity score matched estimates showed a similar trend. A total of nine studies with 157 809 participants were included in the meta-analysis. Higher compared to lower chocolate consumption was associated with significantly lower CHD risk (five studies; pooled RR 0.71, 95% CI 0.56 to 0.92), stroke (five studies; pooled RR 0.79, 95% CI 0.70 to 0.87), composite cardiovascular adverse outcome (two studies; pooled RR 0.75, 95% CI 0.54 to 1.05), and cardiovascular mortality (three studies; pooled RR 0.55, 95% CI 0.36 to 0.83).
Conclusions Cumulative evidence suggests that higher chocolate intake is associated with a lower risk of future cardiovascular events, although residual confounding cannot be excluded. There does not appear to be any evidence to say that chocolate should be avoided in those who are concerned about cardiovascular risk.
Statistics from Altmetric.com
Introduction
Chocolate is an important dietary source of flavonoid antioxidants, which are hypothesised to have a beneficial effect on endothelial function and protect against cardiovascular disease (CVD).1 Evidence from a range of small scale intervention trials have reported that intake of chocolate resulted in increased high density lipoprotein (HDL) cholesterol concentrations, decreased low density lipoprotein (LDL) oxidation, and improved endothelial function.2 Large scale intervention studies have not been performed and therefore the potential benefits of raising chocolate consumption on cardiovascular risk are unknown. However, some evidence on the potential beneficial effects of chocolate can be derived from observational studies.
Many studies have evaluated the risk of cardiovascular outcomes with chocolate consumption.1–4 One meta-analysis which attempted to quantify systematically the effect of high chocolate consumption was limited by inclusion of a heterogeneous spectrum of outcomes including diabetes and heart failure.3 A more recent meta-analysis specifically evaluated the effects of flavonols (also found in other foods) and the risk of coronary heart disease (CHD) and found no significant association between flavonol intake and CHD.4 Many cohort studies which examined the association between chocolate and CVD (defined by CHD and stroke in this study) are limited to sex-specific cohorts.5–9 Two meta-analyses have been performed which pooled many of these studies and found reduced risk of incident stroke7 and CVD10 with higher chocolate consumption. However, these studies are limited because one study only considered stroke while the other study pooled many different cardiovascular events into a single risk estimate.
In order to evaluate any association between habitual chocolate consumption and the risk of cardiovascular events, we analysed data from the European Prospective Investigation into Cancer-Norfolk (EPIC-Norfolk), a large prospective population study in the UK, and incorporated the results from this observational study into the evidence available to date from the literature by conducting a systematic review and meta-analysis.
Methods
EPIC-Norfolk cohort study
EPIC-Norfolk is a prospective population study of over 25 000 men and women, resident in Norfolk, UK (99.6% white Caucasian). The study methods have been described previously in detail.11 In brief, at the baseline survey between 1993 and 1997, participants completed a health and lifestyle questionnaire and provided information on physician-diagnosed chronic diseases such as cancer, myocardial infarction, stroke, and diabetes mellitus. We identified cigarette smoking habit (never, former, current)12 and a validated, self-reported physical activity measure (inactive, moderately inactive, moderately active, active)13 and assessed reported intake of alcoholic drinks (where units (8 g alcohol)/week were categorised into: zero consumption, >0–7, >7–14, >14–21; >21–28; >28 units/week). At the baseline health examination (N=25 639), a trained nurse measured height, weight, and blood pressure (Acutorr). Total cholesterol, HDL cholesterol and triglycerides were measured in non-fasting blood samples as described previously.11 Blood samples were stored at −80°C. LDL cholesterol was calculated by the Friedewald formula. Data on lipids were available for 23 074 participants (90%) and for inflammation markers on minimally 18 643 participants (73%).
Main exposure measure: chocolate consumption
Dietary measurements were obtained by use of the food frequency questionnaire (FFQ), which assessed overall diet in the past year from 24 782 participants (response rate 97%). Every food item or group of food items in the questionnaire had nine answer categories: never or less than once per month, 1–3 per month, once a week, 2–4 per week, 5–6 per week, once a day, 2–3 per day, 4–5 per day, 6+ per day. This instrument and the way it is analysed, using the Compositional Analyses from Frequency Estimates (CAFÉ), are described in detail elsewhere.14
Three questions from the FFQ were considered indicative of chocolate consumption, namely ‘Chocolates singles or squares’ (average portion size of 8 g), ‘Chocolate snack bars, for example, Mars, Crunchie’ (average portion size of 50 g), and ‘Cocoa, hot chocolate (cup)’ (average portion size of 12 g powder weight; the liquid to make up the beverage was hence not included). Frequency categories were multiplied by the portion size to derive the amount of chocolate product eaten (g/day). The sum of the weights of these food items consumed, rather than their flavonoid or cocoa content, formed the measure of exposure.
Clinical outcomes
Participants admitted to hospital were identified using their unique NHS (National Health Service) number by data linkage with ENCORE (East Norfolk Health Authority database). All participants were flagged for death certification at the UK Office of National Statistics, ascertaining vital status for the entire cohort. CHD was defined as ICD-10 (10th Revision of the Internal Classification of Diseases and Related Health Problems) codes I20–25 (which includes the spectrum of CHD including myocardial infarction, unstable angina, and stable angina); stroke was defined as ICD-10 codes I60–69 (which includes ischaemic stroke, haemorrhagic stroke, and stroke of undetermined cause). CVD was defined as the combination of CHD and stroke. A previous validation study in our cohort indicated high specificity for such case ascertainment.15 We report data with follow-up to 31 March 2008, an average of 11.3 years (2.8 SD, median 11.9 years). The study was approved by the Norwich District Health Authority Ethics Committee. All participants provided signed informed consent.
Statistical analysis
Participants without a completed FFQ (n=857), with prevalent myocardial infarction or stroke (n=1102), and whose reported chocolate intake was more than 5 SDs above the median (ie, 100 g/day) were excluded from the analyses (n=86), leaving 23 638 participants eligible; we further excluded 2687 participants who missed data on one or more covariables (n=20 951). Baseline characteristics were calculated by quintiles of chocolate consumption. Since 4195 participants (approximately 20%) reported zero chocolate intake, the lowest quintile corresponds to the non-users only and was used as the reference category in Cox proportional hazards model. HR and corresponding 95% CIs for the risk of future CHD, stroke or CVD were calculated using four models. Regression model 1 adjusted for sex and age. Regression model 2 additionally adjusted for lifestyle factors: smoking, physical activity, energy intake (MJ/day), and alcohol intake (categorical). Regression model 3 adjusted for the variables in model 2 and possible mediators: body mass index (BMI), systolic blood pressure, LDL cholesterol, HDL cholesterol, and prevalent diabetes. In model 4 we additionally adjusted for C-reactive protein (CRP). Analyses were performed using SPSS V.17.0 (Chicago, Illinois, USA).
Systematic review and meta-analysis
To be eligible for inclusion in our review, studies had to report on the association between chocolate consumption and cardiovascular outcomes. We searched PubMed and EMBASE from inception until June 2013 using the terms described in online data supplement 1, with no language limitations, and we checked bibliographies of included articles. In addition to our search of EMBASE (which already includes unpublished abstracts and conference reports) we also searched conference abstracts from the European Society of Cardiology congresses from 2005 to 2013 inclusive. A further search of ISI Web of Science was conducted in February 2014 to identify additional articles. Two reviewers independently screened abstracts and titles, and then obtained full-text versions of potentially relevant studies to confirm eligibility. Data extraction of included studies was performed by CSK as well as JKY, and checked by YKL. Study validity was evaluated based on ascertainment of chocolate consumption and cardiovascular outcomes as well as steps taken to reduce confounding in the primary studies. We pooled data using the inverse variance method and random effects model in RevMan V.5.2 software (Nordic Cochrane Center, Copenhagen, Denmark). For these comparisons, we used the multivariable adjusted measures of association (HRs, relative risks or ORs) for the highest category of chocolate consumption versus the lowest category of consumption. Heterogeneity was estimated using I2, and we considered a value >50% to demonstrate substantial heterogeneity.16 We planned to evaluate publication bias through asymmetry testing if there were >10 studies in the dataset, and no evidence of significant heterogeneity.17
Sensitivity analyses
We performed additional analysis to determine if a similar direction of effect was observed using propensity score matching on the pre-specified covariates of models 1, 2 and 3. Propensity score matching were performed in STATA using the ‘teffects psmatch’ function which estimates treatment effects from observational data.18 The results of the propensity score analysis was used as sensitivity analysis for the meta-analysis. We conducted additional analysis excluding the Djousse study because it was a cross-sectional study.
Results
EPIC-Norfolk cohort
A complete dataset on relevant baseline characteristics were available for 20 951 study participants; 9214 men and 11 737 women (n=16 162 when restricted to participants with CRP data). Mean follow-up was 11.9±2.8 years, and total person years was 236 942 years. A total of 3013 (14.4%) people experienced a fatal or non-fatal CHD event, stroke or both, referred to as CVD. Among these participants with CVD events, 2434 (11.6%) had a CHD event and 848 (4.0%) had a stroke event. When analyses were limited to people with available CRP at baseline, a total of 2207 (13.7%) study participants experienced a CVD event while 1754 (10.9%) and 648 (4.0%) had a CHD event and/or stroke event, respectively.
Chocolate consumption in the EPIC-Norfolk cohort
The median daily chocolate consumption was 4.6 g/day (IQR 0.6–12.0); among consumers only, median chocolate intake was 7.0 g/day (IQR 3.5–15.5). Higher chocolate consumption was associated with trends towards a beneficial cardiovascular risk factor profile including lower age, lower BMI, lower waist/hip ratio, lower systolic blood pressure, lower concentrations of apolipoprotein B (apoB) and CRP, a lower prevalence of diabetes mellitus, and more physical inactivity (table 1). In contrast, higher chocolate consumption was more prevalent among men and among current smokers. Higher chocolate intake was associated with a higher energy intake, with lower contributions from protein and alcohol sources and higher contribution from fat and carbohydrates.
Baseline cardiovascular risk factors by quintiles of chocolate intake in 20 951 men and women of EPIC-Norfolk
Chocolate consumption and risk of CVD in EPIC-Norfolk
Higher chocolate intake was associated with a statistically significant lower risk of CVD, with stronger associations for CVD mortality than for total CVD (hospitalisation or mortality) (table 2). HRs attenuated after adjustment, but remained borderline significant for total CVD (HR 0.89, 95% CI 0.79 to 1.00) and CVD mortality (HR 0.75, 95% CI 0.62 to 0.92). Adjustment for CRP minimally changed the effect estimates. A significant dose–response association was present for both total incidence and CVD mortality. We assessed the proportionality assumption graphically and the results suggest that proportional hazards were maintained with time.
Risk of total (fatal and non-fatal) and fatal CVD incidence by quintiles of chocolate intake in EPIC-Norfolk (1993–2008)
Higher chocolate consumption was associated with a lower risk of hospitalisation or mortality due to CHD in crude and minimally adjusted models (table 3). This association was attenuated after adjustment for a range of cardiovascular risk factors and after additional adjustment for a set of dietary parameters (HR 0.91, 95% CI 0.80 to 1.04). In the smaller sample of participants with CRP data, we observed a significant association with 18% lower risk in quintile 5 versus quintile 1 (95% CI 0.70 to 0.97). Participants with a high chocolate intake also had a lower risk of stroke. The sex- and age-adjusted HR was 0.77 (95% CI 0.62 to 0.96) for top versus bottom quintile of chocolate consumption (table 3). This association remained statistically significant upon adjustment for smoking, physical activity, and dietary variables. Additional adjustment for mediators did not materially change the estimate (HR 0.78, 95% CI 0.63 to 0.98). Analyses using mortality as outcome, rather than combined mortality and hospitalisations, showed lower risk estimates for CVD, CHD, and stroke, although the number of events were substantially lower and therefore the CIs became wider.
Risk of total (fatal and non-fatal) CHD and stroke incidence by quintiles of chocolate intake in EPIC-Norfolk (1993–2008)
Propensity score matched analysis for chocolate consumption and risk of CVD
The baseline characteristics of the unmatched and propensity matched cohorts are shown in online supplementary appendix table 1. A Love plot was used to examine the standardised difference in covariates before and after propensity score adjustment (see online supplementary appendix figure 1). The risk of CHD and stroke by quintiles of chocolate intake considering various levels of adjustments and propensity score matching are shown in online supplementary appendix table 2. After propensity score matching, a trend for benefit with chocolate consumption was apparent for both CHD and stroke, but with lower sample sizes and wider CIs they were not statistically significant.
Systematic review and meta-analysis
Search results and studies of chocolate and CVD
We screened 392 titles and abstracts and identified eight studies5–9 ,19–21 that met eligibility criteria (see online supplementary appendix figure 2). Including the EPIC-Norfolk study, a total of nine studies with 157 809 participants were included in the meta-analysis. These studies included seven cohort studies, one post-hoc analysis of a randomised trial, and one cross-sectional study, and the follow-up duration of the cohort studies ranged from 8–16 years (table 4). Two studies were conducted in the USA and one study was conducted in Australia, but the remainder were conducted in Europe (UK, Netherlands, Germany, Sweden). Three studies included both men and women, but the remainder were sex specific (two studies of men and two studies of women). The mean age of the participants in the included studies ranged from 49–79 years.
Study design, participants, follow-up and outcomes for studies evaluating chocolate consumption and CVD
Bias assessment for studies of chocolate and CVD
Different methods for evaluating and ascertaining chocolate consumption and cardiovascular outcomes were used across the studies (see online supplementary appendix table 3). One study used patient interviews to ascertain chocolate consumption but the remaining studies used questionnaires. The majority of studies used ICD codes to ascertain cardiovascular diagnoses. Five of the studies used linkage of data to mortality registry/records in order to ascertain mortality. All the included studies were able to use a variety of adjustments to account for the effect of confounders; for the meta-analysis, we used data from model 2 of EPIC-Norfolk. While a key cardiovascular risk factor such as BMI was adjusted for in seven studies, other important risk factors such as cholesterol were only considered in two studies (see online supplementary appendix table 4). We considered the cross-sectional study to have lower validity due to the inability to draw a temporal relationship between chocolate use and adverse cardiovascular outcomes.
Chocolate consumption and risk of cardiovascular events
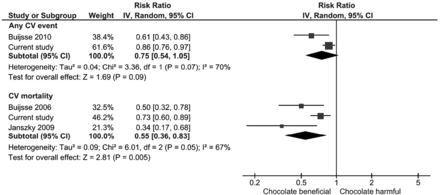
Overall, chocolate consumption was significantly associated with lower risk of CHD across five studies (pooled RR 0.71, 95% CI 0.56 to 0.92; I2=61%) (figure 1). The risk of CHD mortality from one study showed no significant difference with and without chocolate consumption (RR 0.98, 95% CI 0.88 to 1.10). For risk of stroke with chocolate consumption there was significantly lower risk of both stroke incidence (pooled RR 0.79, 95% CI 0.70 to 0.87; I2=0%, five studies) (figure 1) and mortality (RR 0.85, 95% CI 0.74 to 0.98; one study). There was a significant lower risk of any cardiovascular event (pooled RR 0.75, 95% CI 0.54 to 1.05; I2=70%, two studies) and cardiovascular mortality (pooled RR 0.55, 95% CI 0.36 to 0.83; I2=67%, three studies) (figure 2). The propensity matched results were incorporated in the meta-analysis and this continued to show a significant association between chocolate consumption and reduction in CHD (pooled RR 0.72, 95% CI 0.55 to 0.93; I2=63, five studies) and stroke (pooled RR 0.78, 95% CI 0.70 to 0.88; I2=0%, five studies) (figure 3). Publication bias was not assessed because there were fewer than 10 studies included in the analysis.
Meta-analysis risk of the association between chocolate consumption and risk of coronary heart disease (CHD) and stroke.
Meta-analysis of chocolate consumption and risk of cardiovascular (CV) disease (composite).
Meta-analysis risk of coronary heart disease (CHD) and stroke with chocolate consumption using propensity matched cohort.
We conducted additional analysis excluding the Djousse study because it was a cross-sectional study. The lower risk associated with chocolate and CHD was slightly less after exclusion of this study but still remained statistically significant (RR 0.84, 95% CI 0.75 to 0.95 vs RR 0.79, 95% CI 0.70 to 0.87) (data not shown).
Discussion
In this large prospective population study, higher intake of chocolate up to 100 g/day was associated with a lower risk of CVD and stroke, with stronger associations for mortality than total incidence. We have built on the findings of the previous meta-analysis7 ,10 by including this current study in an updated meta-analysis. The reviews by Larsson et al7 only considered stroke as an outcome while the review by Zhang et al10 considered CVD. The current review included stroke, stroke mortality, CHD, and CHD mortality separately. In addition, we included the EPIC-Norfolk cohort which is a Caucasian population. Our results indicate that chocolate consumption was associated with a lower risk of CVD.
A number of issues have to be taken into account when interpreting the results of the present study. Although FFQs are well-established methods to quantify dietary information in large scale population studies, they have limitations: importantly, recall bias as well as underreporting, particularly among women and participants who are obese.22 Underreporting is selective, and includes sweets and snacks. It is possible that lower CVD rates among people who report consuming more chocolate might be due to differential underreporting of chocolate intake in those with potentially greater CVD risk such as the obese and physically inactive. It has been suggested that dark chocolate may have more beneficial effects than milk chocolate.23 Milk chocolate was more frequently consumed than dark chocolate in this cohort (unpublished results); however, we still observed a reduced risk of CVD. This may indicate that not only flavonoids, but also other compounds—possibly related to milk constituents such as calcium and fatty acids—may provide an explanation for the observed association. In addition, the whole dietary pattern may be of relevance, giving concern for unmeasured confounding. Therefore, this observational study cannot provide evidence on the potential causality of the observed association.
We used the FFQ to assess chocolate intake and this measure of dietary assessment method has been validated in the EPIC-Norfolk cohort.24 While the FFQ is prone to recall bias and requires the participant to ‘average out’ over a long period of time, this method has the advantage that it covers a longer time-frame than other methods such as 7-day diet diary (7dDD) and 24 h-diet recall. Chocolate consumption might be a more episodically consumed food for a proportion of the participants and this could have miss-classified the participant as a non-consumer or a high consumer depending in which week the 7dDD or day of 24 h-diet recall would have been completed.
It is possible that part of the observed association could be explained by reverse causality in that people with a higher risk profile, including those with obesity, diabetes mellitus, or prevalent CVD, eat less chocolate-containing foods than people who have a perceived healthy risk profile. However, we excluded people who reported prevalent CVD at the baseline health questionnaire, and analyses were adjusted for diabetes mellitus. We observed that the reference group (non-chocolate consumers) had the highest mean BMI, highest median CRP, highest proportion of participants with diabetes, highest levels of inactivity, and lowest fat intake compared to the other quintiles of chocolate consumers. Alternatively, it may be that higher chocolate consumers have other behaviours that are beneficial for cardiovascular health. Participants with a higher energy intake are also likely to have a higher energy expenditure due to physical activity. They are hence more likely to consume more foods, including chocolate containing foods, which may explain part of the observed associations, although stratified analyses suggested that the association found was homogeneous across levels of physical activity. In addition to multivariable adjustment, we conducted stratified analyses and propensity score matching for baseline differences. Nevertheless we cannot exclude residual confounding from these or other unmeasured factors. The consumption of chocolate as well as its influence on cardiovascular risk may be different depending on the ethnicity of the participants. External validity of our results may be limited to Caucasians.
Our results are somewhat surprising since the expectation was that benefits of chocolate consumption would be mainly associated with dark chocolate rather than the commercially available products generally used in a British population which are high in sugar content and fat. One study has shown reduced incidence of diabetes among men and women with chocolate intake,25 and other studies have shown that chocolate consumption increases bodyweight.26 ,27 This may suggest that there is a balance between benefit and risk with chocolate intake, which is dependent on the risk profile of the individual including baseline weight and dose of chocolate intake.
We did not include heart failure as an outcome for the current analysis for the following reasons. The pathophysiology of CHD and stroke which relates to atherosclerosis differs from that of heart failure. The physiological changes in heart failure are largely driven by neuroendocrine dysregulation and renin–angiotensin–aldosterone system activation. The mechanism by which chocolate might have an impact on these pathways may differ. In addition, the risk factors and confounders in heart failure differ from those in the CVD analysis.
This analysis included clinical biomarkers and anthropometry measured by trained staff. Adjustment for these variables attenuated the association between chocolate consumption and risk of CVD, CHD and stroke, but particularly in the case of stroke and fatal CVD, associations remained significant. CRP was available for a subcohort, in which we observed a better cardiovascular risk profile; however, we observed that CRP did not modify the association between chocolate and CVD, CHD or stroke. Pathways other than anti-inflammatory markers, such as blood pressure, may be a more likely mechanism to explain the observed association between chocolate and CVD/stroke.
Bias may also affect the systematic review if the original studies made post-hoc decisions on particular outcomes or categories for analysis based on the nature of the findings. The primary studies collected data using different categories of chocolate consumption (eg, frequent vs rare, different quintiles or quartiles) and it seemed that some of the studies made post-hoc decisions on what the cut-offs were in defining categories for analysis (including pooling certain categories while excluding others). This raises the possibility of bias from selective outcome and analysis reporting where categorical cut-offs could have been chosen based on the statistical significance of the findings. Nevertheless, the cumulative evidence reported in this study suggests that high chocolate consumption may be associated with cardiovascular benefit.
Future research is still needed to explore the association between chocolate and CVD. Studies are needed to understand better the biological mechanism by which chocolate and flavonoids reduce the risk of CVD. It is also possible that some individuals will not benefit from increased chocolate consumption, such as those who are overweight or diabetic. More research is needed to identify individuals who will benefit most.
In conclusion, our findings support the previously reported association between habitual chocolate intake and a lower risk of CHD events in the large EPIC-Norfolk prospective population study, and we have further set this in context with consistent results seen in meta-analysis of current evidence. While randomised controlled trials of chocolate and cardiovascular end points could be conducted, feasibility is uncertain. For the time being, within the general context of existing recommendations for behaviours conducive to cardiovascular health, there does not appear to be evidence that chocolate should be avoided in terms of impact on cardiovascular risk.
Key messages
What is already known on this subject?
Chocolate consumption has been associated with a lower risk of cardiovascular disease, but studies have limitations with regard to participant selection and combined outcome measures.
What might this study add?
We observed evidence that higher intake of chocolate may be associated with lower cardiovascular disease and mortality.
Our meta-analysis of eight studies found a lower risk of cardiovascular disease with chocolate consumption.
How might this impact clinical practice?
Our study adds to the previously found inverse associations between chocolate intake and risk of future cardiovascular events.
There does not appear to be any evidence to say that chocolate should be avoided in those who are concerned about cardiovascular risk.
Acknowledgments
The authors would like to thank the participants of the EPIC-Norfolk cohort. We thank the nutritionists’ team and data management team of the EPIC-Norfolk cohort.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1 - Online supplement
Press release
Files in this Data Supplement:
Footnotes
CSK and SMB are joint first authors.
PKM and K-TK are joint last authors.
Contributors K-TK and NJW are Principal Investigators of EPIC-Norfolk cohort. SMB analysed the EPIC-Norfolk data. RNL is responsible for data management and linkage and contributed to data analysis. MAHL and SMB designed the cohort analysis. CSK and YKL performed the literature search for systematic review. CSK and JKY screened and collected the data for systematic review which was checked by YKL and PKM. CSK and YKL conducted the meta-analysis. SMB, PKM, CSK and YKL drafted the paper and all authors contributed to the writing of the paper.
Funding The EPIC-Norfolk study was supported by grants from the Medical Research Council and Cancer Research UK.
Competing interests None declared.
Patient consent Obtained.
Ethics approval Norwich District Health Authority Ethics Committee.
Provenance and peer review Not commissioned; externally peer reviewed.