Article Text
Abstract
Objective Familial hypercholesterolaemia (FH) is characterised by elevated low-density lipoprotein (LDL)-cholesterol and increased risk of cardiovascular disease. However, FH remains substantially underdiagnosed and undertreated. We employed a two-stage pragmatic approach to identify and manage patients with FH in primary healthcare.
Methods Medical records for 232 139 patients who attended 15 general practices at least once in the previous 2 years across five Australian States were first screened for potential risk of FH using an electronic tool (TARB-Ex) and confirmed by general practitioner (GP) clinical assessment based on phenotypic Dutch Lipid Clinic Network Criteria (DLCNC) score. Follow-up GP consultation and management was provided for patients with phenotypic FH.
Results A total of 1843 patients were identified by TARB-Ex as at potential risk of FH (DLCNC score ≥5). After GP medical record review, 900 of these patients (49%) were confirmed with DLCNC score ≥5 and classified as high-risk of FH. From 556 patients subsequently clinically assessed by GPs, 147 (26%) were diagnosed with phenotypic FH (DLCNC score >6). Follow-up GP consultation and management for 77 patients resulted in a significant reduction in LDL-cholesterol (−16%, p<0.01). A higher proportion of these patients attained the treatment target of 50% reduction in LDL-cholesterol (74% vs 62%, p<0.001) and absolute levels of LDL-cholesterol goals compared with baseline (26% vs 12%, p<0.05).
Conclusions A pragmatic approach integrating electronic medical record tools and clinical GP follow-up consultation is a feasible method to identify and better manage patients with FH in the primary healthcare setting.
Trial registration number 12616000630415.
- atherosclerosis
- electronic health records
- hyperlipidemias
- global burden of disease
- delivery of healthcare
Data availability statement
Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- atherosclerosis
- electronic health records
- hyperlipidemias
- global burden of disease
- delivery of healthcare
Introduction
Familial hypercholesterolaemia (FH) is a codominantly inherited lipid disorder with over 90% penetrance, principally due to mutations in the low-density lipoprotein (LDL) receptor that causes marked elevations in plasma LDL-cholesterol.1 2 FH has a prevalence of 1 in 250 in the general population.2–4 Lifetime exposure to elevated LDL-cholesterol puts patients at significantly higher risk of atherosclerotic cardiovascular disease (ASCVD).1–3 Despite increasing awareness of the condition,2 5 6 FH remains underdiagnosed and undertreated due to a lack of effective screening and management strategies.2 5–8
FH guidelines support improved primary care-based detection and management.2 5 6 With 88% Australians attending a general practitioner (GP) annually,9 GPs are ideally placed to assist with FH detection and management.10–12 To date, little attention has been given to such an approach in general practice.13
While genetic testing for pathogenic mutations provides a definitive diagnosis of FH,14 assessment tools, such as the Dutch Lipid Clinic Network Criteria (DLCNC), the Simon Broome, FAMCAT and Make Early Diagnosis to Prevent Early Deaths criteria, are widely employed in the clinical setting.5 6 Of these, the DLCNC score remains the most popular diagnostic method in Australia.6 FH screening of electronic health records (EHRs) in primary care can identify patients with high DLCNC score.15–17 We have previously validated a time and cost-effective electronic screening tool (TARB-Ex) to help identify potential risk patients based on their DLCNC score.16 The clinical value of TARB-Ex in facilitating FH detection in general practice, followed by clinical care by GPs, requires further demonstration.
Treatment of elevated LDL-cholesterol is the cornerstone of FH management.5 18 GP-managed lowering of LDL-cholesterol levels in patients with FH through lifestyle modification and drug therapy is critical for reducing risk of ASCVD. The effectiveness of this approach has not been widely investigated in general practice.
We employed a pragmatic approach to detect and manage FH index cases in primary care. We evaluated the yield of detection of patients with FH based on the TARB-Ex tool and clinical follow-up in 15 Australian general practices. We also investigated the effectiveness of GP-managed care in reduction of LDL-cholesterol levels and attainment of therapeutic targets.
Methods
Settings and participants
The study protocol has been published and involves a pragmatic approach using the real-life clinical infrastructure of Australian general practice.19 Briefly, we carried out a non-randomised, non-controlled preintervention and postintervention study to investigate the effectiveness of a two-stage screening programme on the detection and follow-up management of FH in general practice. The study was conducted between 2016 and 2020 in 15 general practices: 5 in Western Australia (WA); 5 in New South Wales (NSW)—1 withdrew prior to completion; 3 in Queensland (QLD); 1 in Victoria (VIC) and 2 in Tasmania (TAS). Eligible practices were Royal Australian College of General Practitioners accredited, used Best Practice software (ie, SNOMED coding system for recording clinical encounters),19 expressed interest in the research topic and included GPs, practice nurses (PNs) and managers willing to be trained and perform DLCNC assessment. The TARB-Ex data extraction tool, validated to operate on Best Practice software, uses an in-built algorithm that corrects LDL-cholesterol levels among patients on lipid-lowering medications (statins with or without ezetimibe).16
Study procedure
The study protocol included four major steps, namely education and training, electronic screening and clinical assessment, follow-up consultation and management as well as patient and public involvement. Full details are available as online supplemental file.
Supplemental material
Statistical analysis
Statistical analyses were performed using SPSS V.25 (IBM, Armonk, New York, USA). Descriptive analyses are presented as mean±SD or number (%) where applicable. We used Shapiro-Wilk test to determine whether variables were normally distributed. Clinical and biochemical variables between patients who completed and did not complete the follow-up consultation and management were compared using independent t-test or χ² test. Yields of patients identified with FH were described as the number of patients required to detect one new case at different stages in the screening process. Effects of GP management on plasma LDL-cholesterol between baseline (the closest treated/untreated LDL-cholesterol level to the first consultation) and lowest follow-up LDL-cholesterol following GP consultation(s), were recorded and compared using Wilcoxon signed-rank test. We carried out mixed model repeated measures analysis to test whether the changes in plasma LDL-cholesterol during follow-up period was significant. Changes in proportion of patients reaching LDL-cholesterol targets between baseline and follow-up were compared using McNemar’s test. Statistical significance was defined as p<0.05.
Results
TARB-Ex screening and medical record review
Figure 1 shows patient flow for TARB-Ex screening, manual record review, clinical assessment and follow-up consultations. A total of 232 139 patients (minimum one visit in past 2 years) attended the practices over study period. Of these, 67 932 patients (29%) were identified by TARB-Ex with a recorded LDL-cholesterol measurement. A total of 1843 patients had DLCNC score ≥5 and classified as potential risk of FH. The prevalence of patients at potential risk was 1:126 of total patients (0.79%) and 1:37 for the subgroup of patients (2.7%) with a cholesterol measurement (1843 out of 232 139 patients and 67 932 patients, respectively). Of 1843 patients with manual medical record reviews by GPs, 900 (49%) were confirmed with DLCNC score ≥5 and classified as high-risk. The prevalence of patients at high-risk of FH was 1:256 of total patients and 1:75 for the subgroup of patients with a cholesterol level recorded (900 out of 232 139 patients and 67 932 patients, respectively).
Flow diagram for TARB-Ex screening, medical record review, clinical assessment and follow-up consultation. DLCNC, Dutch Lipid Clinic Network Criteria; FH, familial hypercholesterolaemia; LDL, low-density lipoprotein.
Clinical assessment of potential cases of FH
A total of 678 patients with high-risk, potential FH (75%) with DLCNC score ≥5 were invited to attend a GP review. The remaining 222 patients (25%) were unable to be contacted. Of the 678 high-risk patients, a detailed clinical assessment was undertaken by GPs on 556 patients, while 122 patients did not attend. A total of 147 patients with DLCNC score ≥6 were diagnosed as phenotypic FH.
Yields of identifying patients with FH
Figure 2 shows yields of 147 patients identified with phenotypic FH at different stages in the study. The yield was 1 in 462 from patients with cholesterol measurement (0.2%) and 1 in 13 from those at potential risk as screened by TARB-Ex (8.0%). GP medical record review identified/classified one new case of FH for every six patients at high-risk (16%) while clinical assessment identified one new case for every four patients who attended GP review (26%).
Yield of detection of 147 patients with phenotypic familial hypercholesterolaemia. FH, familial hypercholesterolaemia; GP, general practitioner.
Follow-up consultation and management
A total of 133 patients with phenotypic FH consented to the study (figure 1). These included 96 newly diagnosed index cases from TARB-Ex screening, 27 patients with existing FH and 10 patients with incidental FH diagnosis (new cases to the practice and/or identified by GP clinical assessment via usual care, not through initial TARB-Ex extraction). All consented patients were invited to join the FH Australasia (FHAN) Registry with 106 included by 30 November 2020.
Table 1 shows baseline clinical and biochemical characteristics of the 133 patients. They were on average middle-aged, overweight, normotensive and mildly hypercholesterolaemic. 58 patients (44%) had at least one clinical CVD event while 113 (85%) had a family history of premature coronary artery disease (CAD). A total of 71 patients (53%) were current (n=19) or ex-smokers (n=52). A total of 87 patients were on statin alone (65%), 7 on ezetimibe alone (5.3%) and 25 on both (19%). The average equivalent dose of atorvastatin was 50.9±26.4 mg/day. The percentage of patients attaining treatment target of 50% reduction in LDL-cholesterol from their highest LDL-cholesterol prior to treatment was 65%. The percentage achieving the absolute target of LDL-cholesterol recommended was 16% (<2.6 mmol/L and <1.8 mmol/L for primary and secondary prevention, respectively).18
Baseline clinical and biochemical characteristics of the 133 patients with phenotypic familial hypercholesterolaemia
From 133 consented patients, 77 attended minimum 1 follow-up GP consultation and had LDL-cholesterol measurement. The remaining 56 patients did not complete the study either failing to attend follow-up consultations (n=29) and/or had no follow-up lipid profile (n=44). There were no significant differences in clinical and biochemical variables between the two groups at recruitment (table 1). Of the 77 patients, 48 had minimum 2 follow-up consultations and 28 had three. The average follow-up period was 221±44 days. A total of 359 first-degree or second-degree relatives were identified from 77 FH index cases (ie, 4.7 relatives/case), as needing cascade screening.
During follow-up, 28 patients (36%) commenced statin therapy (n=5) or changed to higher dose of existing statin (n=22) or received higher potency statin (n=1). The corresponding equivalent dose of atorvastatin increased from 34.3 mg/day to 61.1 mg/day. A total of 20 patients (26%) received GP advice to modify lifestyle factors and 4 (5.2%) required specialist referral. Patients failing to attain LDL-cholesterol target at baseline (50% LDL-cholesterol reduction) were more likely to receive higher intensity treatment than those reaching treatment target (50% vs 29%, p=0.09); however, this did not reach significance. Among 11 patients receiving GP lifestyle advice alone, plasma concentrations of total cholesterol (6.2±0.6 mmol/L vs 5.8±0.5 mmol/L) and LDL-cholesterol (3.6±0.5 mmol/L vs 3.4±0.4 mmol/L) did not significantly differ from baseline (p>0.05 for both).
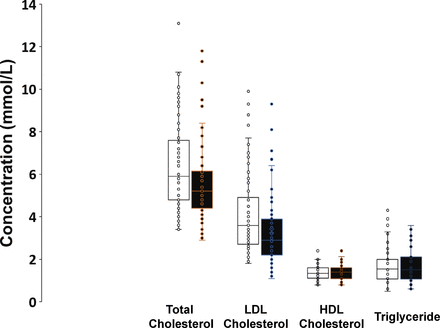
Figure 3 shows plasma concentration of total cholesterol, LDL-cholesterol, high-density lipoprotein (HDL)-cholesterol and triglycerides at baseline and after follow-up. There were significant reductions in plasma total cholesterol (−9.4%) and LDL-cholesterol (−16%) levels in patients with GP consultation/ management compared with baseline (p<0.01). Patients changed to higher dose of existing statin or receiving higher potency statin had greater reduction in LDL-cholesterol levels compared with those without (−24% vs –9.2%, p<0.05; figure 4). Of 77 patients who completed the study, 54 had one follow-up plasma LDL-cholesterol assessment, and 18 had 2 and 5 had 3 assessments. Using mixed model repeated measures analysis, the reduction in plasma LDL-cholesterol levels was significant during follow-up period (p<0.01). The individual changes in plasma LDL-cholesterol levels following GP consultation are shown in online supplemental figure 1.
Plasma lipid concentrations in the 77 patients at baseline and after GP consultations. GP, general practitioner; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Percentages changes in plasma concentration of LDL-cholesterol in patients with or without changing to a higher intensity statin LDL-cholesterol lowering treatment. *Increased intensity of treatment refers to 28 patients started on stain therapy (n=5) or changed to a higher dose of existing stain (n=22) or received a higher potency statin (n=1) for LDL-cholesterol lowering. LDL, low-density lipoprotein.
A higher proportion of patients achieved treatment target of 50% LDL-cholesterol reduction after receiving GP management (74% vs 62%, p<0.001). More GP-managed patients with FH attained the absolute levels of LDL-cholesterol target goals compared with their baseline (26% vs 12%, p<0.05) (figure 5). More specifically, 15 patients with baseline LDL-cholesterol above treatment cut-off attained LDL-cholesterol target after follow-up consultation(s).
Attainment of LDL-cholesterol by a 50% reduction from highest untreated level (A) and an absolute target value for primary and secondary prevention (B) in the 77 patients at baseline and after GP consultation. Baseline value refers to the closest treated/untreated LDL-cholesterol level to the first consultation. Target absolute value of LDL-cholesterol refers to an absolute level of LDL-cholesterol (ie, <2.6 mmol/L or <1.8 mmol/L for primary and secondary prevention).18 GP, general practitioner; LDL, low-density lipoprotein.
Discussion
This study provides a pragmatic approach for improving the detection and management of FH in general practice. Using a two-stage process combining screening electronic data with subsequent clinical assessment, we diagnosed 147 patients with phenotypic FH from a total of 232 139 patients attending 15 GP clinical practices across five Australian States. Importantly, GP management was effective in lowering LDL-cholesterol levels for patients at higher ASCVD risk.
Previous studies
Several approaches have attempted to detect patients with FH in primary care.11 15–17 20–23 including four studies employing electronic data extraction tools.15–17 23 Using combined computer-based and notes-based searches, Grey et al identified 9 new cases of phenotypic FH (DLCNC score >5) in single general practice of 12 100 registered patients.15 Weng et al used FAMCAT, including nine diagnostic risk variables, to identify patients with high FH risk following clinical assessment using specific diagnostic criteria or genetic testing with a predictive accuracy of 86%.23 Using a SQL-based electronic screening tool (TARB-Ex), Troeung et al identified 32 patients at potential FH risk (DLCNC score >5) in one practice of 3708 active patients.16 Kirke et al used data extraction software (Canning Tool) to screen for patients with FH indicators in 2 regional practices of 41 100 EHRs and identified 32 patients with phenotypic FH (DLCNC score >5) after follow-up clinical assessment by trained FH nurse.17 These small scale pilot studies (1–2 general practices) did not examine the effectiveness of subsequent GP management. In a study of 32 patients diagnosed with possible FH, Weng et al found that primary care intervention resulted in small and non-significant reductions in total cholesterol (−2%) and LDL-cholesterol (−3%).24 Our research has extended these studies to an Australia-wide primary care-based programme for detection and management of new FH index cases.
Detection of patients with phenotypic FH
Despite identifying 147 patients with phenotypic FH following GP assessment, over 30% did not undergo clinical assessment because they were not contactable or did not attend. Despite efforts by practices to invite patients at high-risk of FH, low capture rate was constrained by real-world clinical care priority setting. Lack of awareness and knowledge of FH among patients/families may explain their underappreciation of the need to attend such assessment.5 6 8 Further efforts are required to improve public awareness of FH.
Effectiveness of GP management in FH
Given the lifelong risk for ASCVD associated with FH, intensive LDL-cholesterol reduction is required for these patients. While 65% of consented patients attained 50% LDL-cholesterol reduction from untreated highest LDL-cholesterol levels, only 16% attained guideline recommendation of absolute LDL-cholesterol target level of <2.6 mmol/L (or <1.8 mmol/L in adults with ASCVD).5 This observation is consistent with analyses from the FHAN and SAFEHEART registries where only 25% and 11% of patients with FH reached the LDL-cholesterol treatment target, respectively.7 25 Our study identified a similar treatment gap in the primary care setting where currently recommended LDL-cholesterol targets are difficult to achieve for most patients with FH, probably due to inadequate treatment with high-intensity statins and other medications for persistent high LDL-cholesterol.
Evidence supports short-term and long-term benefits of lowering LDL-cholesterol for the prevention of ASCVD among patients with FH.5 6 18 Our research found that implementing a pragmatic intervention plan by GPs (statin/ezetimibe medication±lifestyle advice) resulted in significant reduction in LDL-cholesterol levels. As expected, the greatest LDL-cholesterol reduction was observed in patients changed to higher intensity LDL-cholesterol lowering treatment, resulting in an approximate 1.3 mmol/L decrease in LDL-cholesterol. More importantly, the achieved absolute LDL-cholesterol target level reduction could translate into a 20%–25% relative reduction of global CV risk.18 In addition, we found that the proportion of patients with FH achieving treatment targets significantly increased with GP follow-up management. While the results were encouraging, there remained 74% of patients with FH failing to reach the recommended absolute LDL-cholesterol targets. This underscores the need for more intensive GP management including additional specialist support for greater use of more potent cholesterol-lowering medication (such as proprotein convertase subtilisin/kexin type 9 inhibitors) if applicable. Enhancing GP knowledge of FH and/or awareness of current guidelines to improve the effectiveness of FH management merits further investigation.5 6
More than 40% of patients with FH did not complete the study mainly by failing to attend follow-up consultations. It is difficult to control patient motivation in primary care where a myriad of competing factors including consultation costs take precedence in the mind of the patient. This underscores potential barriers to effective FH management from the patient perspective, including knowledge of FH and perception of ASCVD risk.26 Increasing community awareness of the importance of elevated cholesterol as a risk factor for ASCVD, as well as focused education (especially smoking cessation) for patients/families with FH about this disorder and their high risk of ASCVD, is critically important.
Strengths and limitations
Our study’s strength includes the use of a two-stage process to facilitate an improved detection rate of FH. We used a pragmatic approach to investigate the effect of follow-up GP consultation and management of plasma LDL-cholesterol levels in primary care and piloted the inclusion of patients in the FH registry.5 6
Our study also has limitations. The accuracy of TARB-Ex extractions might have been affected by non-compliance with statin to correctly estimate untreated LDL-cholesterol, together with other factors, including poor quality of medical information in practice EHRs (eg, family history of CAD) and the presence of secondary hypercholesterolaemia, including cholestasis, nephrotic syndrome, steroid use, hypothyroidism. Whether use of age-specificity of untreated LDL-cholesterol levels or another case-finding algorithm could improve specificity for the detection of FH merits further investigation.27 We cannot exclude the possibility that detection of FH could be missed in patients with DLCNC score of 3–4 (possible FH) but this would require assessment of a much larger sample.
The low numbers undertaking follow-up consultations reflect reality in clinical practice and are acknowledged as potential bias in our findings. The pragmatic design of our study also did not allow us to employ a placebo-controlled design to study the effects of GP management on plasma LDL-cholesterol in general practice. Hence, our findings need to be interpreted with caution. Despite GPs and PNs receiving education and training on FH, poor knowledge and awareness of the condition might have impacted detection yield.28 Other barriers, including limited time and resources for training and diagnostic assessment, also need to be considered. The follow-up period for some was relatively short (~7 months) and might not allow enough time to determine the programme’s effectiveness. Variations in compliance with lifestyle advice and medications between patients might have confounded the analysis and merits further investigation. While we identified 359 close relatives as potential targets, cascade testing was not formally implemented. The logistics of undertaking cascade testing in primary care needs further exploration with lack of practice infrastructure seen as a major barrier. The involvement of specialist lipid clinics in family cascade testing through GP referrals is available in major cities but further extension should be considered. Our study did not involve genetic testing and numbers of confirmed phenotypes with a potential mutation remain unknown. Children and people under 18 years of age were not included in the TARB-Ex search and represent another potential missed opportunity to improve FH detection. Whether the improvement in LDL-cholesterol level and change in treatment was due to the behaviour change in patients after their FH diagnosis and face-to-face consultations with GPs, merits further investigation.
Conclusion and future directions
FH is a major public health problem with a need to address the gap in care.2 5–7 Early detection and treatment of FH is recommended to reduce the risk of ASCVD.2 5 6 However, most affected individuals remain undiagnosed and undertreated. While cascade screening is the most cost-effective means of finding new index cases,29 it requires a targeted approach to increase their identification. Using a pragmatic approach with existing infrastructure, our study shows a nationwide, two-stage screening programme based on general practice is feasible. The study also highlights the importance of follow-up consultation and management by GPs in improving treatment in high-risk patients. Further research into improving compliance with lifestyle advice and medications merits investigation.
Several strategies may enhance detection of FH, including universal screening of children, child-parent cascade testing, and screening of patients presenting to coronary care units.5 6 10 22 There is a need to integrate our general practice approach with these strategies to further enhance the detection of FH, particularly in the young. Other challenges include the integration of GP and specialist services.13 30 Health economic evaluation and patient perceptions (ie, outcomes and experience) of our approach will be reported separately. The systematic implementation of a primary case-based cascade testing programme among close relatives of newly identified FH index cases also merits investigation.
Key messages
What is already known on this subject?
Familial hypercholesterolaemia (FH) is an underdiagnosed and undertreated inherited disorder largely due to a lack of effective screening and management strategies for improving care of FH.
General practice is increasingly recommended as an optimal setting for FH screening and management.
What might this study add?
Our study provides a pragmatic approach to identify 147 patients with FH from a total of 232 139 patients attending 15 general practices across five Australian States.
We also demonstrate that follow-up consultation and management is effective in lowering LDL-cholesterol levels by 16% as a consequence of increased intensity in statin therapy and we identified 359 first-degree and second-degree relatives from FH index cases suitable for cascade screening.
How might this impact on clinical practice?
A pragmatic approach integrating electronic medical record tools and clinical GP follow-up consultation is a feasible method to identify and better manage patients with FH in primary healthcare.
Improved infrastructure in primary care is needed to undertake cascade testing of close relatives of FH index patients.
Data availability statement
Data are available on reasonable request.
Ethics statements
Ethics approval
The study was approved by the Human Research Ethics Committee of The University of Notre Dame Australia (Protocol ID 0 16 067F).
Acknowledgments
We thank the staff and patients at the participating general practices for their assistance in the study. Also, to T Grace, L Troeung, W Chan She Ping-Delfos, L Hall, V Foulkes-Taylor, K Holloway-Kew, D Campbell and S Wilks for project management support and to M Bulsara for statistical advice.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors TB conceived, designed and conducted the study, provided methodological support including practice visits, conducted the analyses, interpreted the results and wrote, read and edited the manuscript. DC conducted the analyses, interpreted the results and wrote, read and edited the manuscript. JR, CHeal, GG, CHesp contributed to study design, conducted the study, read and edited the manuscript. CVG, CC, BS helped conduct the study, read and edited the manuscript. DAR, IL, AV and JP contributed to study conception and design, read and edited the manuscript. DS and GW contributed to study conception and design, interpretation of results, provided specialist support, read and edited the manuscript.
Funding The study was supported by the National Health Medical Research Council (NHMRC) partnership grant (GNT1142883). Royal Perth Hospital Medical Research Foundation provided FH registry funding. Mackay Base Hospital Research Funding provided funding for the Queensland arm. WATHN provided support for community conversations in WA. The Western Australia Department of Health provided funding support for study analysis. The WA & QLD study arms were supported by funding from Sanofi-Aventis Australia Pty Ltd (Sanofi). The NSW arm was supported by funding from Amgen Australia Pty Ltd.
Disclaimer Neither Sanofi nor Amgen were involved in the design, collection, analysis, interpretation or reporting of the study, but were given the opportunity to review the manuscript prior to publication. The decision to submit for publication was made independently by the authors. Sanofi and Amgen will be allowed access to all de-identified data from the study for research and audit purposes, if requested.
Competing interests TB has received honoraria for lectures or research grants from Amgen and Sanofi. DAR has received research grants from Sanofi and WA Department of Health, and travel and accommodation support from Amgen. GW has received honoraria for lectures and advisory boards or research grants from Amgen, Arrowhead, AstraZeneca, Esperion, Kowa, Novartis, Regeneron and Sanofi.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Linked Articles
- Editorial
- Cardiac risk factors and prevention