Abstract
Aims/hypothesis
Patients with type 2 diabetes mellitus are at greater cardiovascular risk than the general population. Although it is widely acknowledged that diabetes is a risk factor for coronary artery disease, the increased prevalence of potentially lethal left ventricular abnormalities in this population is less well appreciated.
Methods
We carried out an echocardiographic study of 500 subjects with type 2 diabetes mellitus to assess the prevalence of left ventricular hypertrophy (LVH) and left ventricular systolic dysfunction (LVSD). We also assessed whether abnormalities in diastolic filling parameters were present.
Results
Of the 371 patients in whom left ventricular mass could be successfully assessed, 264 had LVH (71%). Left ventricular systolic dysfunction was much less common, being present in 16/385 patients (4.2%). Long axis contraction was abnormal in 29/429 patients (6.8%). Diastolic filling abnormalities were present in 178/435 (41%) of patients who could be classified using the selected criteria.
Conclusions
We conclude that left ventricular abnormalities are common in type 2 diabetic patients. As medical therapy is available for both LVH and LVSD and has been demonstrated to reduce cardiovascular death, these left ventricular abnormalities could be ideal targets for screening, followed by selective therapeutic intervention.
Similar content being viewed by others
Introduction
The prevalence of diabetes mellitus is increasing, with projections suggesting that, worldwide, the number of adults with diagnosed type 2 diabetes will more than double to 300 million in 2025 [1]. This is of major public health importance since patients with type 2 diabetes are at increased risk of developing and dying from cardiovascular disease, which accounts for up to 70% of deaths in this population [2]. The epidemiology and characteristics of coronary artery disease in type 2 diabetes are well described in the literature [3–7]. The focus in reducing cardiovascular deaths in diabetes tends almost exclusively to be on reducing fresh coronary events. However, abnormalities in the left ventricular structure and function could be equally important contributors to cardiac death in diabetes [8]. Despite this, the prevalence and spectrum of left ventricular abnormalities have not been comprehensively described in a large sample of type 2 diabetic subjects.
It is well established that various left ventricular abnormalities strongly promote cardiac death, in particular left ventricular hypertrophy (LVH) [9–13] and left ventricular systolic dysfunction (LVSD) [14, 15]. Impaired long axis contraction of the left ventricle may also be associated with increased mortality [16]. Furthermore, the presence of left ventricular diastolic filling abnormalities has been demonstrated to place an individual at increased cardiovascular risk [17, 18] and is associated with impaired exercise tolerance [19]. We sought to determine the prevalence of these potentially modifiable left ventricular abnormalities, namely LVH, LVSD, left ventricular long axis contraction and diastolic filling abnormalities, in a cohort of 500 type 2 diabetic subjects.
Methods
Study population
Five hundred volunteers with type 2 diabetes mellitus were randomly recruited from the Diabetes Centre, Ninewells Hospital, Dundee, between April 2002 and October 2003. The only inclusion criterion to be fulfilled was the presence of type 2 diabetes mellitus, defined according to World Health Organization guidelines [20] and ascertained from the Diabetes Centre patient casenotes. The only exclusion criteria were frailty and the inability to give written, informed consent to the study. All subjects who volunteered for the study attended the hospital on one further occasion, during which routine history, examination, ECG and transthoracic echocardiography were performed. Ethical approval was obtained from the Tayside Committee of Medical Research Ethics and all participating subjects gave written, informed consent.
Electrocardiography
A resting 12-lead ECG was recorded for each subject at 10 mm/mV and 25 mm/s with the subject lying supine. ECG left ventricular hypertrophy (ECG LVH) was defined as the presence of either the Sokolow–Lyon criterion or the Cornell voltage product criterion, as used for entry into the Losartan Intervention for Endpoint Reduction in Hypertension study.
Echocardiography
Transthoracic echocardiography was performed by one trained operator (A. Dawson) using a Hewlett-Packard (Andover, MA, USA) Sonos 2500 Phased Array Imaging System with a 2.5 MHz transducer. The scan was performed with the patient lying in the left lateral position at approximately 45°.
Left ventricular hypertrophy assessment
Two-dimensional directed M-mode measurements were made from the parasternal long-axis view just below the tips of the mitral valve. All measurements were made according to the American Society of Echocardiography (ASE) recommendations at end-diastole, taken as the onset of the QRS complex. The leading edge method was used to measure interventricular septal wall thickness, left ventricular internal diameter and left ventricular posterior wall thickness. Measurements were made over at least three separate cardiac cycles and the average taken. Left ventricular mass was calculated according to the formula of Devereux et al. [21] and indexed to height2.7 to give a left ventricular mass index (LVMI). Left ventricular hypertrophy was defined as an LVMI greater than 47 g/m2.7 in women and greater than 50 g/m2.7 in men. Left ventricular mass was also indexed to body surface area and LVH defined as LVMI greater than 110 g/m2 in women and greater than 134 g/m2 in men. LVMI was not calculated in cases in which either poor image quality or inadequate image alignment prevented accurate M-mode measurements from being made.
Left ventricular geometry was classified as normal, concentric remodelling, eccentric left ventricular hypertrophy or concentric left ventricular hypertrophy, based on left ventricular mass and relative wall thickness. Relative wall thickness (RWT) was defined as ([2PWTd]/LVIDd) (PWT is posterior wall thickness, LVID is left ventricular internal diameter) and a value <0.45 was defined as normal. Normal left ventricular geometry was defined as normal left ventricular mass and normal RWT, concentric remodelling defined as normal left ventricular mass and increased RWT, eccentric LVH defined as increased left ventricular mass and normal RWT, and concentric LVH defined as increased left ventricular mass and increased RWT.
Left ventricular systolic function assessment
Quantitative assessment of left ventricular systolic function was made using the modified biplane Simpson’s method to calculate a left ventricular ejection fraction [22]. Three measurements from successive cardiac cycles were made in the two-chamber and four-chamber views. Left ventricular systolic dysfunction was defined as a left ventricular ejection fraction less than 45%.
Assessment of diastolic parameters
Doppler echocardiographic recordings were performed by pulsed-wave Doppler with the sample volume at the tips of the mitral valve leaflets in the apical four-chamber view, in accordance with ASE guidelines. At least three measurements from three consecutive cardiac cycles were made for each parameter. Transmitral recordings were used to measure the peak velocity of early rapid filling (E wave) and peak velocity of atrial filling (A wave), from which the E/A ratio was calculated. E-wave deceleration time was measured as the time interval between the peak of the E-wave and the point at which its descending segment, or its extrapolation, crossed the zero-velocity baseline. Isovolumic relaxation time was the time between aortic valve closure and the onset of diastolic flow. This was assessed in accordance with ASE guidelines by placing a pulsed-wave sample volume between the aortic and mitral valves in the apical five-chamber view, enabling mitral valve inflow and aortic valve outflow signals to be obtained simultaneously.
Age-related threshold values used for defining abnormal E/A ratio, E-wave deceleration time and isovolumic relaxation time were those proposed by the European Study Group on Diastolic Heart Failure [23].
Assessment of long axis contraction
The method of left atrioventricular plane displacement (AVPD) was used to assess long axis contraction of the left ventricle [16]. In the four-chamber view, the 2D-guided M-mode cursor was placed at the lateral region of the atrioventricular plane at the mitral annulus, perpendicular to the direction of movement of the left ventricle, producing an M-mode recording of the atrioventricular (AV) plane displacement. The vertical distance between the point of the AV plane most distant from the apex and the point closest to the apex was measured in the M-mode. This procedure was repeated at the septal region of the AV plane in the four-chamber view, and the inferior and anterior regions of the AV plane in the two-chamber view. At least three measurements were made from each region, giving 12 measurements from which the mean left AVPD was calculated. A mean value of less than 10 mm was taken as an abnormal AVPD.
Statistics
Values are quoted as means and 95% confidence intervals. A minimum of three measurements was used to calculate the mean for each parameter. Comparisons of continuous variables between groups were performed with the independent samples t-test. Comparisons between categorical variables were performed using the chi-square test. Standard multiple regression analysis was performed to establish which variables were independently related to left ventricular mass. All statistical analyses were performed using SPSS for Windows version 11.0. A value of p<0.05 was considered to be statistically significant.
Results
Patient characteristics of the 500 subjects studied are listed in Table 1.
Left ventricular hypertrophy
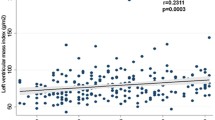
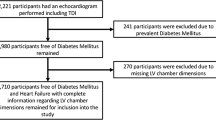
A left ventricular mass index was obtainable in 371/500 subjects (74%); those in whom left ventricular mass could not be assessed only differed significantly from those in whom an adequate echocardiographic image could be obtained in age (66.3 [95% CI 64.5–68.1] vs 63.0 [95% CI 61.9–64.1] years, respectively, p=0.002). The proportion of subjects with LVH was 43% (159/371) when left ventricular mass was indexed to body surface area and 71% (264/371) when left ventricular mass was indexed to height2.7. Tables 2 and 3 show the characteristics of patients with and without left ventricular hypertrophy. Multivariate analysis was performed to include all possible determinants of left ventricular mass index: sex, age, BMI, duration of diabetes, smoking status, systolic BP, diastolic BP, creatinine and HbA1c. This model explained only 18.4% of the variation in LVMI, and the factors independently related to LVMI were BMI (standardised β=0.401, p<0.001), age (standardised β=0.209, p=0.001) and sex (standardised β=0.170, p<0.004). Patients with LVH were significantly more likely to be prescribed calcium channel antagonists (51 vs 22%, p=0.028), diuretics (37 vs 16%, p=0.026) and oral nitrates (13 vs 4%, p=0.025) than patients without LVH. The distribution of left ventricular geometry in the studied population (n=371) is shown in Figs. 1 and 2: the majority of LVH was eccentric rather than concentric, regardless of whether left ventricular mass was indexed to body surface area or to height2.7. The results were almost identical if a cut-off value for RWT of 0.43 (as used in other studies) was used instead of 0.45 (results not shown).
Left ventricular systolic dysfunction
An ejection fraction was obtainable in 385/500 subjects (77%); those in whom the ejection fraction could not be assessed differed from those in whom it could be assessed only in BMI (32.4 [95% CI 31.4–33.4] and 28.9 [95% CI 28.4–29.4] kg/m2 respectively, p<0.001) and in sex (43% male vs 67% female, p<0.001). The prevalence of left ventricular systolic dysfunction was 4% (16/385). Table 4 shows the characteristics of subjects with and without LVSD. Multivariate analysis was performed including sex, age, BMI, duration of diabetes, smoking status, systolic BP, diastolic BP, creatinine and HbA1c. This model explained 11.8% of the variation in ejection fraction. The factors independently related to ejection fraction were sex (standardised β=–0.248, p<0.001), BMI (standardised β=–0.132, p=0.027), age (standardised β=–0.141, p=0.033) and systolic blood pressure (standardised β=–0.152, p=0.029).
Left ventricular diastolic abnormalities
The prevalence of abnormal diastolic function was 40.9% in the 435 subjects that could be classified in this way (Electronic Supplementary Material [ESM], Table 1). There were no significant differences in baseline characteristics between those in whom an assessment of diastolic function could or could not be made. Left ventricular mass index was significantly higher in males with diastolic abnormalities but not in females. Multivariate analysis was not performed.
Left ventricular long axis contraction
AVPD was obtained in 429/500 patients (85.8%). Patients in whom AVPD could not be assessed were more likely to be female and had higher BMI than those in whom AVPD could be assessed. The prevalence of AVPD <10 mm was only 6.8% (29/429). As expected, those with an AVPD <10 mm were significantly older, with lower left ventricular ejection fractions and more diastolic abnormalities (ESM, Table 2). Multivariate analysis was performed, including sex, age, BMI, duration of diabetes, smoking status, systolic BP, diastolic BP, creatinine and HbA1c. This model explained 28.7% of the variation in AVPD and the factors independently related to AVPD were sex (standardised β=0.478, p<0.001) and BMI (standardised β=0.286, p<0.001).
Cardiac rhythm
Four hundred and eighty-four of 500 (96.8%) patients were in sinus rhythm; 2.8% of patients (14/500) were in atrial fibrillation. One patient (0.2%) had paced rhythm and one patient had second-degree heart block (0.2%).
Discussion
The two main left ventricular abnormalities assessed in our population of type 2 diabetic subjects were LVH and LVSD. Our primary finding is that there is a very high prevalence of LVH in subjects with type 2 diabetes (43–71%) and a somewhat lower prevalence of LVSD (4%). Our other main findings are that 41% of type 2 diabetic subjects had abnormalities in the diastolic parameters tested and 6.8% had abnormal left ventricular long axis contraction.
Left ventricular hypertrophy is an independent predictor of cardiovascular death that is currently rather ignored. This is despite the fact that in one head-to-head study, LVH was a bigger risk factor for death (relative risk 2.4) than left ventricular systolic dysfunction (relative risk 2.0) or multivessel coronary artery disease (relative risk 1.6) [24]. In addition, the cardiovascular risk associated with LVH can be reduced by LVH regression [25] and risk returns to normal if full LVH regression is achieved [26, 27]. For these reasons, detection of LVH and targeted intervention to normalise or reduce left ventricular mass could be a promising way of reducing cardiovascular mortality in patients with diabetes. The first step towards achieving this is to assess the prevalence of unsuspected LVH in routine diabetic patients, and this has not been addressed by any other large study. Early studies showed that diabetes is indeed associated with increased left ventricular mass [28], but there is no previous large study of its prevalence in a group of routine diabetic clinic patients. We therefore assessed the epidemiology of left ventricular abnormalities in type 2 diabetic patients to see whether therapeutic opportunities to reduce the high death rates in diabetics could be identified by routine echocardiography of all diabetic patients.
LVH has, until now, been ignored because of two common misconceptions. The first is that LVH only occurs in severe hypertension. Considerable evidence exists to refute this. In our study, prevailing systolic or diastolic BP did not predict LVH in type 2 diabetics, although there was a slight excess of a history of hypertension (8–13%) in those with LVH as opposed to those without, but this was significant for one of the left ventricular mass parameters only. In Framingham, LVH occurred in 28% of women over 60 years with a systolic BP of 125–139 mm Hg [29]. Furthermore, evidence now suggests that BP explains only 25% of the variability in left ventricular mass [30]. Obesity and insulin resistance have been implicated in the pathogenesis of non-hypertensive LVH. Obesity has been demonstrated to be an independent predictor of left ventricular chamber size, left ventricular wall thickness and left ventricular mass [31, 32]. Insulin has been demonstrated to have trophic effects on cardiomyocytes in cell culture and may act as a growth factor, promoting the development of LVH [33]. It has also been suggested that, rather than acting on the heart directly, insulin may influence cardiac structure by stimulating the sympathetic nervous system [34].
The second misconception regarding LVH is that ACE inhibitors are a cure for LVH. This is not the case, as illustrated by both the Left Ventricular Hypertrophy Indapamide Versus Enalapril (LIVE) study [35], in which diuretics were better at reducing left ventricular mass than ACE inhibitors, and also by the Heart Outcomes Prevention Evaluation (HOPE) study [36]. In the LIVE study, treatment with indapamide for 48 weeks significantly reduced left ventricular mass index in hypertensive patients with LVH, whereas enalapril did not. This result could not be accounted for by differences in BP reduction as the magnitude of BP reduction was equivalent for the two treatments. The HOPE study demonstrated the benefits of ramipril in preventing or regressing ECG LVH in patients at high cardiac risk, nearly 40% of whom had diabetes mellitus. There was a relative reduction of approximately 40% in cardiovascular death in those patients in whom ECG LVH was prevented or regressed, compared with those in whom ECG LVH developed or progressed (3.4 vs 5.7%, p=0.001) [37]. The effect of ramipril on LVH was independent of the effect of ramipril on BP reduction. Although this risk reduction seems impressive, LVH itself increases mortality by 150–680% [12] and ACE inhibitors therefore reduce the risk of LVH but do not fully abolish it.
Left ventricular mass is a graded risk factor [38] and it can be argued that dichotomising LVH into being either present or absent is somewhat artificial and dependent upon the cut-off points used for its classification. Furthermore, there is debate as to whether left ventricular mass should be indexed to body surface area or to height. Previous work has demonstrated that the prevalence of LVH in obese populations is underestimated by indexing left ventricular mass to body surface area [39]. For this reason, we indexed left ventricular mass to both height2.7 and body surface area, and found a difference of 28% in LVH prevalence between the two. Both methods have been prognostically validated: in one comparison between them, the risk ratio for a future cardiovascular event was considerably higher (4.06 CI 2.0–8.2) for the height2.7 parameter than for the body surface area parameter (2.76 CI 1.4–5.5) [40]. However, there is a larger body of evidence linking the body surface area parameter to prognosis and it is still widely used. Inevitably there will be debate over which method of assessing LVH is better, but the key point is that the prevalence of LVH in type 2 diabetes is worryingly high (43 or 71%) whichever indexing method is used.
The majority of patients in our study with LVH had eccentric left ventricular geometry. Studies have suggested that concentric LVH may be associated with a higher risk of stroke, cardiac death and all-cause mortality than eccentric LVH, although this is controversial [41–43]. There was a relatively poor correlation between structural LVH and functional diastolic abnormalities in our study, with only around one-half of patients with diastolic abnormalities also having LVH, and vice versa. Previous work has established that diastolic abnormalities occur early in the course of diabetic cardiomyopathy and prevalence estimates of between 30 and 61% have been reported for asymptomatic left ventricular diastolic dysfunction [19, 44, 45]. Our results are supported by those of the Strong Heart Study, in which diastolic abnormalities in diabetic patients were found to be independent of left ventricular mass and left ventricular systolic function [46]. The mechanisms contributing to the pathogenesis of diastolic dysfunction in some diabetic patients, but not others, are not fully established, although some pointers do exist. Firstly, diastolic dysfunction has been shown to be associated with aortic stiffness in patients with diabetes mellitus with no coronary artery disease [47]. Secondly, altered diastolic function in type 2 diabetes is associated with reduced myocardial metabolism, assessed using magnetic resonance imaging [48]. It is possible that the degree of hyperglycaemia may also play a role, as a higher fasting glucose and glycated haemoglobin are associated with abnormal left ventricular relaxation in diabetic patients [46].
The mean systolic BP of patients in our cohort did not meet the target for diabetic patients of 130 mm Hg. However, according to the results of the European Action on Secondary Prevention by Intervention to Reduce Events study [49], this is a fairly typical scenario with cardiac risk factor control remaining suboptimal in most patients in the real world. Perhaps being aware that a patient had LVH (or increased left ventricular mass) would motivate the prescribing physician to intensify cardiac risk factor control in these patients. This is given credence by a recent study demonstrating that doctors do optimise risk factor control in patients at high risk when risk scores are available during the consultation [50]. It is perhaps surprising that, in our study, only 35% of patients were receiving an ACE inhibitor at the time of the study visit, particularly when 61% of our patients had known hypertension. Part of this may be that LVH had not been identified in most of the patients prior to this study. In addition, most evidence suggests that the choice of drug used to lower BP is less important than the actual BP lowering achieved. Such a view was endorsed even for diabetes by the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial study, which was designed to determine whether a calcium channel antagonist (amlodipine), an ACE inhibitor (lisinopril) or an alpha blocker (doxazosin) would prevent the primary outcome of fatal CHD or non-fatal myocardial infarction significantly more than diuretic therapy [51]. Perhaps unexpectedly, there was no significant difference in the primary outcome between the four groups. In terms of secondary outcomes, chlorthalidone was superior to lisinopril in preventing stroke, heart failure, angina and coronary revascularisation. Another reason for the low use of ACE inhibitors in our study may be that many of our patients had been established on alternative antihypertensives long before our study started and prior to the suggestion that ACE inhibitors might be preferable in diabetes to reduce microalbuminuria.
One limitation of our study is the fact that 24% of the patients screened did not have adequate echocardiographic images to allow M-mode measurements of left ventricular dimensions to be made. This is an inevitable limitation of all echocardiographic studies and imaging failure rates of 2–40% have been reported [11, 26, 52–54]. As expected, the two main contributors to inadequate echo images in our study were increasing age and high BMI. If anything, it is likely that such patients would be at greater risk of having LVH, and our figures may therefore underestimate the prevalence of LVH in the diabetic population. A further limitation of our study is that we did not seek to formally exclude silent myocardial ischaemia in our subjects, and it is therefore possible that some may have had significant obstructive coronary artery disease that was not yet clinically apparent and which may have contributed to the prevalence of left ventricular abnormalities. Our study also had no control group, in that we did not compare the prevalence of LVH in patients with and without diabetes and it could be suggested that our high prevalence of LVH may be due to hypertension. However, one study of patients with and without hypertension found the prevalence of LVH to be 27% in hypertensives (defined as BP>160/95) and 6% in normotensives [55]. The prevalence of LVH in our diabetic population was much higher than this, even allowing for the fact that 61% had a history of hypertension.
Type 2 diabetes is a major cause of cardiovascular morbidity and mortality and its prevalence is rising rapidly. Our study provides important information by demonstrating that there is a high prevalence of left ventricular abnormalities in this group. It is possible that routine screening for these abnormalities followed by targeted intervention may help reduce cardiovascular morbidity and mortality. Future work should be aimed at assessing the cost-effectiveness of screening diabetic patients for LVH and then optimising their treatment. As diabetic patients have higher cardiovascular death rates than non-diabetic patients, it may be more cost-effective to target LVH in the former, rather than in the latter. A recent hypothetical analysis of the cost-effectiveness of identifying LVH, performed by Witham et al., suggested that this may indeed be a very cost-effective strategy to reduce cardiovascular events in high-risk normotensive patients, such as diabetics [56].
Abbreviations
- ASE:
-
American Society of Echocardiography
- AV:
-
atrioventricular
- AVPD:
-
atrioventricular plane displacement
- ECG LVH:
-
electrocardiographic left ventricular hypertrophy
- HOPE:
-
Heart Outcomes Prevention Evaluation
- LIVE:
-
Left Ventricular Hypertrophy Indapamide Versus Enalapril
- LVH:
-
left ventricular hypertrophy
- LVMI:
-
left ventricular mass index
- LVSD:
-
left ventricular systolic dysfunction
- RWT:
-
relative wall thickness
References
King H, Aubert RE, Herman WH (1998) Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 21:1414–1431
Laakso M (1999) Hyperglycaemia and cardiovascular disease in type 2 diabetes. Diabetes 48:937–942
Goraya TY, Leibson CL, Palumbo PJ et al (2002) Coronary atherosclerosis in diabetes mellitus. A population-based autopsy study. J Am Coll Cardiol 40:946–953
Dortimer AC, Shenoy PN, Shiroff RA et al (1978) Diffuse coronary artery disease in diabetic patients. Fact or fiction? Circulation 57:133–136
Woodfield S, Lundergan C, Reiner J et al (1996) Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol 28:1661–1669
Falcone C, Nespoli L, Geroldi D et al (2003) Silent myocardial ischemia in diabetic and nondiabetic patients with coronary artery disease. Int J Cardiol 90:219–227
Rytter L, Troelsen S, Beck-Nielsen H (1985) Prevalence and mortality of acute myocardial infarction in patients with diabetes. Diabetes Care 8:230–234
Struthers AD, Morris AD (2002) Screening for and treating left-ventricular abnormalities in diabetes mellitus: a new way of reducing cardiac deaths. Lancet 359:1430–1432
Sullivan JM, Zwaag RV, El-Zeky F, Ramanathan KB, Mirvis DM (1993) Left ventricular hypertrophy: effect on survival. J Am Coll Cardiol 22:508–513
Lorell BH, Carabello BA (2000) Left ventricular hypertrophy. Pathogenesis, detection, and prognosis. Circulation 102:470–479
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322:1561–1566
Vakilli BA, Okin PM, Devereux RB (2001) Prognostic implications of left ventricular hypertrophy. Am Heart J 141:334–341
Kannel WB, Abbott RD (1986) A prognostic comparison of asymptomatic left ventricular hypertrophy and unrecognized myocardial infarction: the Framingham Study. Am Heart J 111:391–397
Lauer M, Evans J, Levy D (1992) Prognostic implications of subclinical left ventricular dilatation and systolic dysfunction in men free of overt cardiovascular disease (the Framingham Heart Study). Am J Cardiol 70:1180–1184
McDonagh TA, Cunningham AD, Morrison CE et al (2001) Left ventricular dysfunction, natriuretic peptides, and mortality in an urban population. Heart 86:21–26
Willenheimer R, Cline C, Erhardt L, Israelsson B (1997) Left ventricular atrioventricular plane displacement: an echocardiographic technique for rapid assessment of prognosis in heart failure. Heart 78:230–236
Schillaci G, Pasqualini L, Verdecchia P et al (2002) Prognostic significance of left ventricular diastolic dysfunction in essential hypertension. J Am Coll Cardiol 39:2005–2011
Møller JE, Søndergaard E, Seward JB, Appleton CP, Egstrup K (2000) Ratio of left ventricular peak E-wave velocity to flow propagation velocity assessed by color M-mode Doppler echocardiography in first myocardial infarction. Prognostic and clinical implications. J Am Coll Cardiol 35:363–370
Poirier P, Bogarty P, Garneau C, Marois L, Dumesnil J (2001) Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes. Diabetes Care 24:5–10
World Health Organization (1999) Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO Consultation. Part 1: diagnosis and classification of diabetes mellitus. Department of Noncommunicable Disease Surveillance, World Health Organization, Geneva, pp 1–59
Devereux RB, Alonso DR, Lutas EM et al (1986) Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 57:450–458
Schiller NB, Shah PM, Crawford M, for the American Society of Echocardiography Committee on Standards, Sub-Committee on Quantitation of Two Dimensional Echocardiograms et al (1989) Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. J Am Soc Echocardiogr 2:358–367
European Study Group on Diastolic Heart Failure (1998) How to diagnose diastolic heart failure. Eur Heart J 19:990–1003
Liao Y, Cooper RS, McGee DL, Mensah GA, Ghali JK (1995) The relative effects of left ventricular hypertrophy, coronary artery disease, and ventricular dysfunction on survival among black adults. JAMA 273:1592–1597
Verdecchia P, Schillaci G, Borgioni C et al (1998) Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation 97:48–54
Muiesan M, Salvetti M, Rizzoni D, Castellano M, Donato F, Agabiti-Rosei E (1995) Association of change in left ventricular mass with prognosis during long-term antihypertensive treatment. J Hypertens 13:1091–1095
Koren MJ, Ulin RJ, Koren AT, Laragh JH, Devereux RB (2002) Left ventricular mass change during treatment and outcome in patients with essential hypertension. Am J Hypertens 15:1021–1028
Galderisi M, Anderson KM, Wilson PWF, Levy D (1991) Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (The Framingham Heart Study). Am J Cardiol 68:85–89
Levy D, Anderson KM, Savage DD, Kannel WB, Christiansen JC, Castelli WP (1988) Echocardiographically detected left ventricular hypertrophy: prevalence and risk factors. The Framingham Heart Study. Ann Intern Med 108:7–13
Devereux R, Pickering T, Harshfield G et al (1983) Left ventricular hypertrophy in patients with hypertension: importance of blood pressure response to regularly recurring stress. Circulation 68:470–476
Okin PM, Jern S, Devereux RB, Kjeldsen SE, Dahlof B (2000) Effect of obesity on electrocardiographic left ventricular hypertrophy in hypertensive patients. The Losartan Intervention For Endpoint (LIFE) reduction in hypertension study. Hypertension 35:13–18
Karason K, Sjöström L, Wallentin I, Peltonen M (2003) Impact of blood pressure and insulin on the relationship between body fat and left ventricular structure. Eur Heart J 24:1500–1505
Hill D, Millner D (1985) Insulin as a growth factor. Pediatr Res 19:879–886
Reaven GM, Lithell H, Landsberg L (1996) Hypertension and associated metabolic abnormalities-the role of insulin resistance and the sympathoadrenal system. N Engl J Med 334:374–382
Gosse P, Sheridan DJ, Zannad F et al (2000) Regression of left ventricular hypertrophy in hypertensive patients treated with indapamide SR 1.5 mg vs enalapril 20 mg: the LIVE study. J Hypertens 18:1465–1475
Heart Outcomes Prevention Evaluation (HOPE) Study Investigators (2000) Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 355:253–259
Mathew J, Sleight P, Lonn E for the Heart Outcomes Prevention Evaluation (HOPE) Investigators et al (2001) Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation 104:1615–1621
Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F (2000) Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 35:580–586
Wachtell K, Bella JN, Liebson PR et al (2000) Impact of different partition values on prevalences of left ventricular hypertrophy and concentric geometry in a large hypertensive population: the LIFE study. Hypertension 35:6–12
de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA, Laragh JH (1995) Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol 25:1056–1062
Ghali JK, Liao Y, Cooper RS (1998) Influence of left ventricular geometric patterns on prognosis in patients with or without coronary artery disease. J Am Coll Cardiol 31:1635–1640
Di Tullio MR, Zwas DR, Sacco RL, Sciacca RR, Homma S (2003) Left ventricular mass and geometry and the risk of ischemic stroke. Stroke 34:2380–2384
Krumholz HM, Larson M, Levy D (1995) Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol 25:879–884
Zabalgoitia M, Ismaeil MF, Anderson L, Maklady FA (2001) Prevalence of diastolic dysfunction in normotensive, asymptomatic patients with well-controlled type 2 diabetes mellitus. Am J Cardiol 87:320–323
Andersen N, Poulsen S, Eiskjær H, Poulsen P (2003) Decreased left ventricular longitudinal contraction in normotensive and normoalbuminuric patients with type II diabetes mellitus: a Doppler tissue tracking and strain rate echocardiography study. Clin Sci 105:56–59
Liu JE, Palmieri V, Roman MJ et al (2001) The impact of diabetes on left ventricular filling pattern in normotensive and hypertensive adults: the Strong Heart Study. J Am Coll Cardiol 37:1943–1949
Eren M, Gorgulu S, Uslu N, Celik S, Dagdeviren B, Tezel T (2004) Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart 90:37–43
Diamant M, Lamb H, Groeneveld Y et al (2003) Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol 42:328–335
EUROASPIRE I and II Group (2001) Clinical reality of coronary prevention guidelines: a comparison of EUROASPIRE I and II in nine countries. Lancet 357:995–1001
Hall L, Jung R, Leese G (2003) Controlled trial of effect of documented cardiovascular risk scores on prescribing. BMJ 326:251–252
The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group (2002) Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 288:2981–2997
Koren M, Devereux R, Casale P, Savage D, Laragh J (1991) Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med 114:345–352
Schirmer H, Lunde P, Rasmussen K (1999) Prevalence of left ventricular hypertrophy in a general population: the Tromso Study. Eur Heart J 20:429–438
Devereux RB, Palmieri V, Liu JE et al (2002) Progressive hypertrophy regression with sustained pressure reduction in hypertension: the losartan intervention for endpoint study. J Hypertens 20:1445–1450
Kuroda T, Shiina A, Tsuruda KT et al (1991) Assessment of hypertensive heart by 2-dimensional echocardiography in mass screening. Jpn Circ J 55:365–376
Witham MD, Davies JI, Dawson A, Davey PG, Struthers AD (2004) Hypothetical economic analysis of screening for left ventricular hypertrophy in high-risk normotensive populations. QJM 97:87–93
Acknowledgements
We would like to acknowledge the British Heart Foundation for providing funding for A. Dawson.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Dawson, A., Morris, A.D. & Struthers, A.D. The epidemiology of left ventricular hypertrophy in type 2 diabetes mellitus. Diabetologia 48, 1971–1979 (2005). https://doi.org/10.1007/s00125-005-1896-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-005-1896-y