Abstract
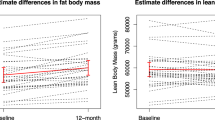
Aromatase inhibitors (AIs) have become the standard adjuvant therapy of postmenopausal breast cancer survivors. AIs induce a reduction of bioavailable estrogens by inhibiting aromatase, which would be expected to induce alterations in body composition, more extensive than induced by menopause. The objectives are to examine the impact of AIs on (1) DXA-scan derived body composition and (2) gonadal hormone levels. This is a sub-analysis of a 2-year double-blind, placebo-controlled, randomized trial of 82 women with nonmetastatic breast cancer, newly menopausal following chemotherapy, who were randomized to risedronate (35 mg once weekly) versus placebo, and stratified for their usage of AI versus no AI. Outcomes included DXA-scan derived body composition and gonadal hormone levels. As a group, total body mass increased in women over 24 months. Women on AIs gained a significant amount of lean body mass compared to baseline as well as to no-AI users (P < 0.05). Women not on an AI gained total body fat compared to baseline and AI users (P < 0.05). Free testosterone significantly increased and sex hormone binding globulin (SHBG) significantly decreased in women on AIs compared to no AIs at 24 months (P < 0.01) while total estradiol and testosterone levels remained stable. Independent of AI usage, chemotherapy-induced postmenopausal breast cancer patients demonstrated an increase of total body mass. AI users demonstrated maintenance of total body fat, an increase in lean body mass and free testosterone levels, and a decrease in SHBG levels compared to no-AI users. The mechanisms and implications of these changes need to be studied further.


Similar content being viewed by others
References
Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9(1):R6
Smith IE, Dowsett M (2003) Aromatase inhibitors in breast cancer. N Engl J Med 348(24):2431–2442
Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M et al (2010) Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 28(3):509–518
Carr MC (2003) The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88(6):2404–2411
Greenspan SL, Brufsky A, Lembersky BC, Bhattacharya R, Vujevich KT, Perera S, Sereika SM, Vogel VG (2008) Risedronate prevents bone loss in breast cancer survivors: a 2-year, randomized, double-blind, placebo-controlled clinical trial. J Clin Oncol 26(16):2644–2652
Fuerst T, Genant HK (1996) Evaluation of body compositlon and total bone mass with the hologic QDR 4500. Osteo Int 6(Suppl 1):203
Lee CC, Kasa-Vubu JZ, Supiano MA (2003) Differential effects of raloxifene and estrogen on insulin sensitivity in postmenopausal women. J Am Geriatr Soc 51(5):683–688
Francucci CM, Daniele P, Iori N, Camilletti A, Massi F, Boscaro M (2005) Effects of raloxifene on body fat distribution and lipid profile in healthy post-menopausal women. J Endocrinol Invest 28(7):623–631
Tommaselli GA, Di Carlo C, Di Spiezio Sardo A, Bifulco G, Cirillo D, Guida M, Capasso R, Nappi C (2006) Serum leptin levels and body composition in postmenopausal women treated with tibolone and raloxifene. Menopause 13(4):660–668
Jacobsen DE, Samson MM, Schouw YT, Grobbee DE, Verhaar HJ (2008) Efficacy of tibolone and raloxifene for the maintenance of skeletal muscle strength, bone mineral density, balance, body composition, cognitive function, mood/depression, anxiety and quality of life/well-being in late postmenopausal women >/=70 years: study design of a randomized, double-blind, double-dummy, placebo-controlled, single-center trial. Trials 9:32
Nguyen MC, Stewart RB, Banerji MA, Gordon DH, Kral JG (2001) Relationships between tamoxifen use, liver fat and body fat distribution in women with breast cancer. Int J Obes Relat Metab Disord 25(2):296–298
Grey A, Stapleton J, Evans M, Reid I (1995) The effect of the anti-estrogen tamoxifen on cardiovascular risk factors in normal postmenopausal women. J Clin Endocrinol Metab 80(11):3191–3195
Francini G, Petrioli R, Montagnani A, Cadirni A, Campagna S, Francini E, Gonnelli S (2006) Exemestane after tamoxifen as adjuvant hormonal therapy in postmenopausal women with breast cancer: effects on body composition and lipids. Br J Cancer 95(2):153–158
Enns DL, Tiidus PM (2010) The influence of estrogen on skeletal muscle: sex matters. Sports Med 40(1):41–58
Yates RA, Dowsett M, Fisher GV, Selen A, Wyld PJ (1996) Arimidex (ZD1033): a selective, potent inhibitor of aromatase in postmenopausal female volunteers. Br J Cancer 73(4):543–548
Bajetta E, Zilembo N, Dowsett M, Guillevin L, Di Leo A, Celio L, Martinetti A, Marchiano A, Pozzi P, Stani S et al (1999) Double-blind, randomised, multicentre endocrine trial comparing two letrozole doses, in postmenopausal breast cancer patients. Eur J Cancer 35(2):208–213
Bhasin S, Woodhouse L, Storer TW (2001) Proof of the effect of testosterone on skeletal muscle. J Endocrinol 170(1):27–38
Miller KK (2009) Androgen deficiency: effects on body composition. Pituitary 12(2):116–124
Ling S, Komesaroff PA, Sudhir K (2009) Cardiovascular physiology of androgens and androgen testosterone therapy in postmenopausal women. Endocr Metab Immune Disord Drug Targets 9(1):29–37
Torrens JI, Sutton-Tyrrell K, Zhao X, Matthews K, Brockwell S, Sowers M, Santoro N (2009) Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: study of Women’s Health Across the Nation. Menopause 16(2):257–264
Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K (2008) Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med 168(14):1568–1575
Ding EL, Song Y, Manson JE, Hunter DJ, Lee CC, Rifai N, Buring JE, Gaziano JM, Liu S (2009) Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med 361(12):1152–1163
Perry JR, Weedon MN, Langenberg C, Jackson AU, Lyssenko V, Sparso T, Thorleifsson G, Grallert H, Ferrucci L, Maggio M et al (2010) Genetic evidence that raised sex hormone binding globulin (SHBG) levels reduce the risk of type 2 diabetes. Hum Mol Genet 19(3):535–544
Abdulhaq H, Geyer C (2008) Safety of adjuvant endocrine therapy in postmenopausal women with breast cancer. Am J Clin Oncol 31(6):595–605
Ewer MS, Gluck S (2009) A woman’s heart: the impact of adjuvant endocrine therapy on cardiovascular health. Cancer 115(9):1813–1826
Gandhi S, Verma S (2007) Aromatase inhibitors and cardiac toxicity: getting to the heart of the matter. Breast Cancer Res Treat 106(1):1–9
Lewis S (2007) Do endocrine treatments for breast cancer have a negative impact on lipid profiles and cardiovascular risk in postmenopausal women? Am Heart J 153(2):182–188
Seruga B, Tannock IF (2009) Up-front use of aromatase inhibitors as adjuvant therapy for breast cancer: the emperor has no clothes. J Clin Oncol 27(6):840–842
Janssen I, Powell LH, Kazlauskaite R, Dugan SA (2009) Testosterone and visceral fat in midlife women: the Study of Women’s Health Across the Nation (SWAN) fat patterning study. Obesity 18(3):604–610
Jacobsen D, Samson M, Emmelot M, Verhaar H (2009) Raloxifene and body composition and muscle strength in postmenopausal women: a randomized, double-blind, placebo-controlled trial. Eur J Endocrinol 162:371–376
Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A (2010) History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev 30(4):343–375
Acknowledgments
All funding sources supporting publication of a work or study are as follows. NIH/NIDDKD (K24: DK062895-05): awarded to Dr. Greenspan. A Procter and Gamble and Sanofi-Aventis noncompany-sponsored trial grant: awarded to Dr. Greenspan. NIH/NCRR (M01-RR00056): awarded to the University of Pittsburgh. NIH/NCRR (RR024154): awarded to Dr. Steven E. Reis. John A. Hartford foundation (2004-0485): provided support for assays. None of these funding agencies were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript. Risedronate, matching placebo, calcium, and vitamin D supplements were provided by Procter and Gamble, Inc. We are indebted to the nursing, professional, laboratory, dietary, administrative, and study staff of the Clinical Translational Research Center of Montefiore University Hospital and Osteoporosis Prevention and Treatment Center at the University of Pittsburgh. We acknowledge the members of the Data and Safety Monitoring Board for their oversight of the study.
Conflict of interest
Dr Greenspan has received grant-support from Procter and Gamble, Inc., Sanofi-Aventis, Amgen, and Lilly. Dr Greenspan also serves as a consultant for Merck. Dr. Perera has received funding in the past from Eli Lilly and Co., Ortho Biotech, LLC, Teva Neuroscience for observational research. All other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Londen, G.J., Perera, S., Vujevich, K. et al. The impact of an aromatase inhibitor on body composition and gonadal hormone levels in women with breast cancer. Breast Cancer Res Treat 125, 441–446 (2011). https://doi.org/10.1007/s10549-010-1223-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-010-1223-2