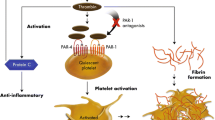
Abstract
In acute coronary syndromes (ACS), a dual antiplatelet regimen with an adenosine diphosphate (ADP) receptor antagonist plus aspirin has become the cornerstone of treatment. The third-generation thienopyridine prasugrel and the cyclopentyl-triazolo-pyrimidine ticagrelor provide a greater, more rapid and consistent platelet inhibition compared to their predecessor clopidogrel. Based on their advantages over clopidogrel in two landmark studies, both drugs received a class I recommendation for their use in ACS patients with and without ST segment elevation. Due to differences in ACS populations and conditions investigated, the relative merits of ticagrelor versus prasugrel in the treatment of ACS patients with planned invasive strategy cannot be reliably estimated from independent trials. To date, no direct head-to-head comparison of ticagrelor and prasugrel in terms of clinical outcome exists. The aim of this multicenter, randomized, open-label trial is to assess whether ticagrelor is superior to prasugrel in ACS patients with planned invasive strategy.

Similar content being viewed by others
Abbreviations
- ACS:
-
Acute coronary syndrome
- ADP:
-
Adenosine diphosphate
- ASA:
-
Acetylsalicylic acid
- CABG:
-
Coronary artery bypass graft
- ISAR-REACT:
-
Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment
- LBBB:
-
Left bundle branch block
- MRI:
-
Magnetic resonance imaging
- NSTE–ACS:
-
Non-ST segment elevation acute coronary syndrome
- NSTEMI:
-
Non-ST segment elevation myocardial infarction
- PCI:
-
Percutaneous coronary intervention
- STEMI:
-
ST segment elevation myocardial infarction
- URL:
-
Upper reference limit
References
Schomig, A., Neumann, F. J., Kastrati, A., et al. (1996). A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. The New England Journal of Medicine, 334, 1084–1089.
Hamm, C. W., Bassand, J. P., Agewall, S., et al. (2011). ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). European Heart Journal, 32, 2999–3054.
Task Force on the management of STseamiotESoC, Steg, P. G., James, S. K., et al. (2012). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. European Heart Journal, 33, 2569–2619.
Kushner, F. G., Hand, M., Smith, S. C., Jr., et al. (2009). 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology, 54, 2205–2241.
Levine, G. N., Bates, E. R., Blankenship, J. C., et al. (2011). 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Journal of the American College of Cardiology, 58, e44–e122.
Gurbel, P. A., Bliden, K. P., Hiatt, B. L., & O'Connor, C. M. (2003). Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation, 107, 2908–2913.
Serebruany, V. L., Steinhubl, S. R., Berger, P. B., Malinin, A. I., Bhatt, D. L., & Topol, E. J. (2005). Variability in platelet responsiveness to clopidogrel among 544 individuals. Journal of the American College of Cardiology, 45, 246–251.
Bonello, L., Tantry, U. S., Marcucci, R., et al. (2010). Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. Journal of the American College of Cardiology, 56, 919–933.
Wiviott, S. D., Braunwald, E., McCabe, C. H., et al. (2007). Prasugrel versus clopidogrel in patients with acute coronary syndromes. New England Journal of Medicine, 357, 2001–2015.
Wallentin, L., Becker, R. C., Budaj, A., et al. (2009). Ticagrelor versus clopidogrel in patients with acute coronary syndromes. The New England Journal of Medicine, 361, 1045–1057.
Brandt, J. T., Payne, C. D., Wiviott, S. D., et al. (2007). A comparison of prasugrel and clopidogrel loading doses on platelet function: magnitude of platelet inhibition is related to active metabolite formation. American Heart Journal, 153(66), 9–16.
Gurbel, P. A., Bliden, K. P., Butler, K., et al. (2009). Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation, 120, 2577–2585.
Sugidachi, A., Asai, F., Ogawa, T., Inoue, T., & Koike, H. (2000). The in vivo pharmacological profile of CS-747, a novel antiplatelet agent with platelet ADP receptor antagonist properties. British Journal of Pharmacology, 129, 1439–1446.
Husted, S., Emanuelsson, H., Heptinstall, S., Sandset, P. M., Wickens, M., & Peters, G. (2006). Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonist AZD6140 with aspirin in patients with atherosclerosis: a double-blind comparison to clopidogrel with aspirin. European Heart Journal, 27, 1038–1047.
Wallentin, L. (2009). P2Y(12) inhibitors: differences in properties and mechanisms of action and potential consequences for clinical use. European Heart Journal, 30, 1964–1977.
Schomig, A. (2009). Ticagrelor—is there need for a new player in the antiplatelet-therapy field? The New England Journal of Medicine, 361, 1108–1111.
Steg, P. G., James, S., Harrington, R. A., et al. (2010). Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: a platelet inhibition and patient outcomes (PLATO) trial subgroup analysis. Circulation, 122, 2131–2141.
Montalescot, G., Wiviott, S. D., Braunwald, E., et al. (2009). Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet, 373, 723–731.
Montalescot, G., Bolognese, L., Dudek, D., et al. (2011). A comparison of prasugrel at the time of percutaneous coronary intervention or as pretreatment at the time of diagnosis in patients with non-ST-segment elevation myocardial infarction: design and rationale for the ACCOAST study. American Heart Journal, 161, 650–656. e1.
Montalescot, G., Bolognese, L., Dudek, D., et al. (2013). Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. New England Journal of Medicine, 369, 999–1010.
Roe, M. T., Armstrong, P. W., Fox, K. A., et al. (2012). Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. The New England Journal of Medicine, 367, 1297–1309.
James, S. K., Roe, M. T., Cannon, C. P., et al. (2011). Ticagrelor versus clopidogrel in patients with acute coronary syndromes intended for non-invasive management: substudy from prospective randomised PLATelet inhibition and patient Outcomes (PLATO) trial. BMJ, 342, d3527.
Held, C., Asenblad, N., Bassand, J. P., et al. (2011). Ticagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery: results from the PLATO (Platelet Inhibition and Patient Outcomes) trial. Journal of the American College of Cardiology, 57, 672–684.
Headrick, J. P., Peart, J. N., Reichelt, M. E., & Haseler, L. J. (1808). Adenosine and its receptors in the heart: regulation, retaliation and adaptation. Biochimica et Biophysica Acta, 2011, 1413–1428.
Serebruany, V. L. (2011). Adenosine release: a potential explanation for the benefits of ticagrelor in the PLATelet inhibition and clinical outcomes trial? American Heart Journal, 161, 1–4.
Ohman, J., Kudira, R., Albinsson, S., Olde, B., & Erlinge, D. (2012). Ticagrelor induces adenosine triphosphate release from human red blood cells. Biochemical and Biophysical Research Communications, 418, 754–758.
Björkman JA, K. I., & van Giezen, J. J. J. (2007). AZD6140 inhibits adenosine uptake into erythrocytes and enhances coronary blood flow after local ischemia or intracoronary adenosine infusion. Circulation, 116(II), 28. Abstract 245.
van Giezen, J. J., Sidaway, J., Glaves, P., Kirk, I., & Bjorkman, J. A. (2012). Ticagrelor inhibits adenosine uptake in vitro and enhances adenosine-mediated hyperemia responses in a canine model. Journal of Cardiovascular Pharmacology and Therapeutics, 17, 164–172.
Wittfeldt, A., Emanuelsson, H., Brandrup-Wognsen, G., et al. (2013). Ticagrelor enhances adenosine-induced coronary vasodilatory responses in humans. Journal of the American College of Cardiology, 61(7), 723–7. doi:10.1016/j.jacc.2012.11.032.
Mehran, R., Rao, S. V., Bhatt, D. L., et al. (2011). Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation, 123, 2736–2747.
Cutlip, D. E., Windecker, S., Mehran, R., et al. (2007). Clinical end points in coronary stent trials: a case for standardized definitions. Circulation, 115, 2344–2351.
Thygesen, K., Alpert, J. S., Jaffe, A. S., et al. (2012). Third universal definition of myocardial infarction. European Heart Journal, 33, 2551–2567.
Wiviott, S. D., White, H. D., Ohman, E. M., et al. (2013). Prasugrel versus clopidogrel for patients with unstable angina or non-ST-segment elevation myocardial infarction with or without angiography: a secondary, prespecified analysis of the TRILOGY ACS trial. Lancet, 382, 605–613.
Sarafoff, N., Martischnig, A., Wealer, J., et al. (2013). Triple therapy with aspirin, prasugrel, and vitamin k antagonists in patients with drug-eluting stent implantation and an indication for oral anticoagulation. Journal of the American College of Cardiology, 61, 2060–2066.
Funding
Currently there is no extramural funding for the trial.
Conflict of interest
Dominick J. Angiolillo reports receiving payment (a) as consulting fee or honorarium from Bristol Myers Squibb, Sanofi-Aventis, Eli Lilly, Daiichi Sankyo, The Medicines Company, AstraZeneca, Merck, Evolva, Abbott Vascular and PLx Pharma; (b) for participation in review activities from Johnson & Johnson, St. Jude, and Sunovion; institutional payments for grants from Bristol Myers Squibb, Sanofi-Aventis, GlaxoSmithKline, Otsuka, Eli Lilly, Daiichi Sankyo, The Medicines Company, AstraZeneca, Evolva; and for other financial relationships with Esther and King Biomedical Research Grant. David Antoniucci reports receiving consulting fee or honorarium from AstraZeneca and Eli Lilly, payment for lectures including service on speakers bureaus for Bayer, and travel/accommodations/meeting expenses unrelated to activities listed from Terumo. Adnan Kastrati reports receiving lecture fees from Abbott, Biotronik and The Medicines; from advisory board meetings for AstraZeneca, MSD, and St. Jude Medical; and from event adjudication for Biosensors. Franz-Josef Neumann reports consultancy, grants/grant pending, and payment for lectures including service on speakers bureaus from Eli Lilly, Daiichi Sankyo, and AstraZeneca. Helmut Schühlen reports consultancy and payment for lectures including service on speakers bureaus from Lilly and Daiichi Sankyo and grants/grants pending from AstraZeneca. The other coauthors have reported no conflicts of interest.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Associate Editor Emanuele Barbato oversaw the review of this article
Author list includes all members of the Steering and Operations Committees in alphabetic order except the principal investigator and study chair.
Clinical trial registration information: URL www.clinicaltrials.gov
Unique identifier NCT01944800
Appendices
Appendix 1: Endpoint Definitions
Death
The primary endpoint includes death from any cause. In addition, the cause of death will be adjudicated. All deaths will be assumed cardiovascular in nature unless a noncardiovascular cause can be clearly provided (e.g., malignancy, trauma, and infection). If an autopsy has been performed, autopsy reports should be solicited for determination of cause of death [1].
Myocardial infarction
The definition of myocardial infarction used in this trial is adapted from the Third Universal Definition of Myocardial Infarction [32]. Cardiac troponin will be used as the preferred biomarker. Creatine kinase-myocardial band (CK-MB) and CK values will be assessed concurrently and used in the case that troponin values are not available.
Spontaneous myocardial infarction (not related to PCI or CABG)
Detection of a rise and/or fall in cardiac biomarkers (preferably cardiac troponin) with at least one value above the 99th percentile upper reference limit (URL) and with at least one of the following:
-
symptoms of ischemia
-
development of pathological Q waves in the ECG
-
new or presumed new ST segment–T wave changes (ST–T changes) or new left bundle branch block (LBBB)
-
imaging evidence of new loss of viable myocardium or new regional wall motion abnormality
Myocardial infarction after randomization and before PCI
Recurrent symptoms of cardiac ischemia or hemodynamic instability plus one of the following criteria:
-
new or presumed new ST segment elevation or new LBBB (distinct from the last ECG)
-
in patients with normal biomarkers and not presenting with STEMI on admission: detection of a rise and/or fall in cardiac biomarkers (preferably cardiac troponin) with at least one value above the 99th percentile URL
-
if the baseline troponin values are elevated and are stable or falling, then a rise of >20 % is required
-
development of new pathological Q waves in the ECG distinct from the coronary territory identified on admission
-
imaging evidence of new loss of viable myocardium or new regional wall motion abnormality
PCI-related myocardial infarction (within 48 h after PCI)
Cardiac enzymes (troponin T or I, CK, and CK-MB) will be determined on admission, in patients with nonurgent PCI again at 4–6 h after admission (before angiography) and from blood drawn from the arterial sheath in the cath lab immediately after sheath insertion and before PCI.
The biomarker course will be used for redefinition of baseline status in patients with NSTE–ACS, i.e., to differentiate unstable angina pectoris from NSTEMI and to better describe biomarker course in NSTEMI patients.
Based on the mandatory two sets of biomarkers (on admission and in the cath lab), the baseline status will be redefined:
-
If biomarkers on admission have been normal (initial diagnosis of unstable angina) and biomarkers are rising >99th percentile URL in the second sample (before catheterization or from the arterial sheath) without recurrent symptoms of ischemia, then the initial diagnosis of unstable angina is revised to ongoing NSTEMI on admission.
-
If biomarkers on admission have been elevated (NSTEMI), then it will be documented whether biomarker values are stable, rising, or falling.
Unstable angina at baseline
In patients undergoing PCI with normal (<99th percentile URL) baseline troponin concentrations, PCI related myocardial infarction requires elevation of troponin >5 × 99th percentile URL occurring within 48 h of the procedure, plus either one of the following:
-
evidence of prolonged ischemia (>20 min) as demonstrated by prolonged chest pain or
-
ischemic ST-changes or new pathological Q waves, or
-
angiographic evidence of a flow-limiting complication, such as of loss of patency of a side branch, persistent slow flow or no reflow, embolization, or
-
imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.
In patients with recent symptoms (<6 h) before admission, no second blood sample before catheterization, a short interval from biomarker assessment on admission and in the cath lab, and normal values in both samples, it will be challenging to differentiate an ongoing myocardial infarction from post-PCI myocardial infarction.
In this case, the diagnosis of myocardial infarction requires criteria as defined in the following “NSTEMI with rising biomarkers or STEMI” section for patients with rising biomarkers.
NSTEMI with documented stable or falling biomarkers
If baseline troponin values are elevated on admission and are stable or falling, then a rise of >20 % is required for the diagnosis of reinfarction. In addition, either of the following is required:
-
symptoms suggestive of myocardial ischemia or hemodynamic instability, or
-
new ischemic ECG changes or new LBBB, or
-
angiographic loss of patency of a major coronary artery or a side branch or persistent slow or no flow or embolization, or
-
imaging demonstration of new loss of viable myocardium or new regional wall motion abnormality.
NSTEMI with rising biomarkers or STEMI
-
new symptoms suggestive of myocardial ischemia or hemodynamic instability plus
-
new ischaemic ECG changes or new LBBB plus
-
angiographic loss of patency of a major coronary artery or a side branch or persistent slow or no flow or embolization
Myocardial infarction related to CABG
Myocardial infarction associated with CABG is defined by elevation of cardiac biomarker values >10 × 99th percentile URL in patients with normal baseline troponin values (<99th percentile URL) in addition to either of the following:
-
new pathological Q waves or new left bundle branch block, or
-
angiographic documented new graft or new native coronary artery occlusion, or
-
imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.
Stroke
Stroke is defined as the new onset of focal or global neurological deficit caused by ischemia or hemorrhage within or around the brain and lasting for more than 24 h or leading to death. The diagnosis of stroke requires confirmation by CT, MRI, or autopsy.
Appendix 2: Study Organizational Structure
Steering Committee: A. Kastrati (Chairman), S. Schulz (Coordinating investigator), D. J. Angiolillo, D. Antoniucci, C. Hamm, K.-L. Laugwitz, F.-J. Neumann, G. Richardt, H. Schühlen, H. Schunkert
Operations Committee: A. Kastrati, S. Schulz, I. Bernlochner, J. Jaitner, K. Mayer, B. von Merzljak, T. Morath, J. Ruf, G. Schömig
Data Coordinating Center: ISAResearch Center Munich: K. Hoesl, H. Holle, J. Neudecker, I. Zenullahi, H. Paul, N. Rifatov, J. Vogel
Data and Safety Monitoring Board: A. Schömig (Chair), F. Hofmann, K. Ulm (Statistician), B. Höfling
Trial Statistician: I. C. Rondak
Event Adjudication Committee: K. Tiroch (Chair), C. Volmer, D. Keta
Angiographic Core Laboratory: R.A. Byrne, S. Pinieck, S. Hurt
ECG Core Laboratory: S. Kufner
MRI Core Laboratory: M. Hadamitzky, S. Cassese
Platelet Function Core Labs: S. Braun, G. Mößmer
Rights and permissions
About this article
Cite this article
Schulz, S., Angiolillo, D.J., Antoniucci, D. et al. Randomized Comparison of Ticagrelor versus Prasugrel in Patients with Acute Coronary Syndrome and Planned Invasive Strategy—Design and Rationale of the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 Trial. J. of Cardiovasc. Trans. Res. 7, 91–100 (2014). https://doi.org/10.1007/s12265-013-9527-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-013-9527-3