Abstract
Calcific aortic stenosis in the elderly is the number one cause of surgical valve replacement in the US and Europe. The incidence of calcific aortic stenosis is increasing as the general age of the population increases. For many years, rheumatic heart disease was the main cause of aortic valve disease. Over the last half century, however, there has been a change from a rheumatic etiology to a 'degenerative' mechanism because of the increase in access to health care in developed countries and the increasing age of the population in the US and Europe. For many years 'degenerative' aortic stenosis was thought to be caused by the passive accumulation of calcium on the surface of the aortic valve leaflet. Recent studies have demonstrated, however, that the etiology of aortic valve disease has a similar pathophysiology to that of vascular atherosclerosis, and that the treatment of this disease could be similar to that of chronic vascular atherosclerosis. This Review will discuss our current understanding of the pathophysiology, risk factors, cellular mechanisms, diagnosis and finally, future medical therapies for calcific aortic stenosis.
Key Points
-
Calcific aortic stenosis is the number one indication for surgical valve replacement in the US and Europe
-
Risk factors include male sex, hypertension, elevated levels of LDL cholesterol, and smoking—similar to those for vascular atherosclerosis
-
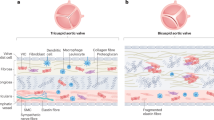
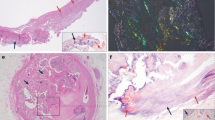
Calcification is known to cause the development of stenosis in the diseased aortic valves
-
Experimental models have demonstrated the critical features of aortic valve calcification—osteoblast expression, cell proliferation and atherosclerosis—and specific bone-cell phenotypes have been found in calcifying human valves
-
The definitive, but not curative, therapy for calcific aortic stenosis is valve replacement
-
Future insights into the mechanisms of calcification and its progression might indicate lipid-lowering therapy in modifying the rate of progression of stenosis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lindroos M et al. (1993) Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol 21: 1220–1225
Ross J Jr and Braunwald E (1968) Aortic stenosis. Circulation 38 (Suppl 1): S61–S67
Rahimtoola SH (1991) Perspectives on valvular heart disease: update II. In An era in cardiovascular medicine, 45–70 (Eds Knoebel SB and Dack S) New York: Elsevier
Roberts WC and Ko JM (2005) Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 111: 920–925
Stewart BF et al. (1997) Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 29: 630–634
Aronow WS et al. (1987) Correlation of serum lipids, calcium, and phosphorus, diabetes mellitus and history of systemic hypertension with presence or absence of calcified or thickened aortic cusps or root in elderly patients. Am J Cardiol 59: 998–999
Otto CM et al. (1999) Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 341: 142–147
Pohle K et al. (2001) Progression of aortic valve calcification: association with coronary atherosclerosis and cardiovascular risk factors. Circulation 104: 1927–1932
O'Brien KD et al. (1996) Apolipoproteins B, (a), and E accumulate in the morphologically early lesion of 'degenerative' valvular aortic stenosis. Arterioscler Thromb Vasc Biol 16: 523–532
Olsson M et al. (1999) Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler Thromb Vasc Biol 19: 1218–1222
Otto CM et al. (1994) Characterization of the early lesion of 'degenerative' valvular aortic stenosis: histological and immunohistochemical studies. Circulation 90: 844–853
Sprecher DL et al. (1984) Cardiovascular features of homozygous familial hypercholesterolemia: analysis of 16 patients. Am J Cardiol 54: 20–30
Rajamannan NM et al. (2003) Hypercholesterolemic aortic-valve disease. N Engl J Med 349: 717–718
Rajamannan NM et al. (2001) Experimental hypercholesterolemia induces apoptosis in the aortic valve. J Heart Valve Dis 10: 371–374
Rajamannan NM et al. (2002) Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation 105: 2660–2665
Garg V et al. (2005) Mutations in NOTCH1 cause aortic valve disease. Nature 437: 270–274
Ortlepp JR et al. (2001) The vitamin D receptor genotype predisposes to the development of calcific aortic valve stenosis. Heart 85: 635–638
Avakian SD et al. (2001) Apolipoproteins AI, B, and E polymorphisms in severe aortic valve stenosis. Clin Genet 60: 381–384
O'Brien KD et al. (1995) Osteopontin is expressed in human aortic valvular lesions. Circulation 92: 2163–2168
Liebe V et al. (2006) Coagulation activation is associated with interleukin-6 plasma levels in patients with mechanical prosthetic heart valves. In Vivo 20: 427–430
Galante A et al. (2001) C-reactive protein is increased in patients with degenerative aortic valvular stenosis. J Am Coll Cardiol 38: 1078–1081
Soini Y et al. (2003) Angiogenesis is involved in the pathogenesis of nonrheumatic aortic valve stenosis. Hum Pathol 34: 756–763
Rajamannan NM et al. (2005) Calcified rheumatic valve neoangiogenesis is associated with vascular endothelial growth factor expression and osteoblast-like bone formation. Circulation 111: 3296–3301
Jian B et al. (2003) Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg 75: 457–465
Kaden JJ et al. (2005) Tumor necrosis factor alpha promotes an osteoblast-like phenotype in human aortic valve myofibroblasts: a potential regulatory mechanism of valvular calcification. Int J Mol Med 16: 869–872
O'Brien KD et al. (2002) Association of angiotensin-converting enzyme with low-density lipoprotein in aortic valvular lesions and in human plasma. Circulation 106: 2224–2230
Chalajour F et al. (2004) Angiogenic activation of valvular endothelial cells in aortic valve stenosis. Exp Cell Res 298: 455–464
Mohler ER et al. (1991) Development and progression of aortic valve stenosis: atherosclerosis risk factors—a causal relationship? A clinical morphologic study. Clin Cardiol 14: 995–999
Rajamannan NM et al. (2003) Calcific aortic stenosis: from bench to the bedside—emerging clinical and cellular concepts. Heart 89: 801–805
Rajamannan NM et al. (2003) Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107: 2181–2184
Mohler ER III et al. (2001) Bone formation and inflammation in cardiac valves. Circulation 103: 1522–1528
Caira FC et al. (2006) Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol 47: 1707–1712
Kaden JJ et al. (2004) Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis 13: 560–566
Mohler ER III et al. (1997) Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol 17: 547–552
Mathieu P et al. (2005) Calcification of human valve interstitial cells is dependent on alkaline phosphatase activity. J Heart Valve Dis 14: 353–357
Rahimtoola SH (2006) The year in valvular heart disease. J Am Coll Cardiol 47: 427–439
Johnson ML and Rajamannan N (2006) Diseases of Wnt signaling. Rev Endocr Metab Disord 7: 41–49
Rajamannan NM et al. (2005) Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation 112 (Suppl 9): I229–I234
Shao JS et al. (2005) Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest 115: 1210–1220
Rosenhek R et al. (2000) Predictors of outcome in severe asymptomatic aortic stenosis. N Engl J Med 343: 611–617
O'Brien KD et al. (2004) Hemodynamic effects of the angiotensin-converting enzyme inhibitor, ramipril, in patients with mild to moderate aortic stenosis and preserved left ventricular function. J Investig Med 52: 185–191
O'Brien KD et al. (2005) Angiotensin-converting enzyme inhibitors and change in aortic valve calcium. Arch Intern Med 165: 858–862
Rajamannan NM et al. (2005) Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart 91: 806–810
American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons; Bonow RO et al. (2006) ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation 114: e84–e231
Aronow WS et al. (2001) Association of coronary risk factors and use of statins with progression of mild valvular aortic stenosis in older persons. Am J Cardiol 88: 693–695
Novaro GM et al. (2001) Effect of hydroxymethylglutaryl coenzyme A reductase inhibitors on the progression of calcific aortic stenosis. Circulation 104: 2205–2209
Bellamy MF et al. (2002) Association of cholesterol levels, hydroxymethylglutaryl coenzyme-A reductase inhibitor treatment, and progression of aortic stenosis in the community. J Am Coll Cardiol 40: 1723–1730
Shavelle DM et al. (2002) HMG CoA reductase inhibitor (statin) and aortic valve calcium. Lancet 359: 1125–1126
Cowell SJ et al. (2005) A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med 352: 2389–2397
Moura LM et al. (2007) Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 49: 554–561
Rossebo A et al. (2003) 3P-0870 Design of the Simvastatin and Szetimide in Aortic Stenosis (SEAS) Study [abstract]. Atheroscler Suppl 4: 253
Rahimtoola SH (2004) Aortic valve disease. In Hurst's The Heart Manual of Cardiology, 1643–1679 (Eds Fuster V et al.) New York: McGraw-Hill Medical
Wood P (1956) Aortic stenosis. In Diseases of the Heart and Circulation, 568–583 (Ed Wood P) London: Eyre and Spootiswoode
Beauchesne LM et al. (2003) Temporal variations in effective orifice area during ejection in patients with valvular aortic stenosis. J Am Soc Echocardiogr 16: 958–964
Leatham A (1951) The phonocardiogram of aortic stenosis. Br Heart J 13: 153–158
Rahimtoola SH (1999) “Prophylactic” valve replacement for mild aortic valve disease at time of surgery for other cardiovascular disease?...No. J Am Coll Cardiol 33: 2009–2015
Rahimtoola SH (2000) Severe aortic stenosis with low systolic gradient: the good and bad news. Circulation 101: 1892–1894
Monin JL et al. (2003) Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 108: 319–324
Griffith MJ et al. (1989) Inaccuracies in using aortic valve gradients alone to grade severity of aortic stenosis. Br Heart J 62: 372–378
Bucciarelli-Ducci C et al. (2006) Contrast-enhanced cardiac magnetic resonance in the evaluation of myocardial infarction and myocardial viability in patients with ischemic heart disease. Curr Probl Cardiol 31: 128–168
Rahimtoola SH (1989) The hibernating myocardium. Am Heart J 117: 211–221
Rahimtoola SH et al. (2006) Hibernating myocardium: another piece of the puzzle falls into place. J Am Coll Cardiol 47: 978–980
Rahimtoola SH (2003) Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol 41: 893–904
Rahimtoola SH (2005) The year in valvular heart disease. J Am Coll Cardiol 45: 111–12255
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
NM Rajamannan is an inventor on a patent for the medical treatment of valvular heart disease. The Mayo Clinic is the owner of the patent and she does not receive any Royalties from these patents.
RO Bonow is a consultant for Edwards Lifesciences in the development of percutaneous valve replacement and repair devices.
SH Rahimtoola declared he has no competing interests.
Rights and permissions
About this article
Cite this article
Rajamannan, N., Bonow, R. & Rahimtoola, S. Calcific aortic stenosis: an update. Nat Rev Cardiol 4, 254–262 (2007). https://doi.org/10.1038/ncpcardio0827
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0827
This article is cited by
-
Association between evolocumab use and slow progression of aortic valve stenosis
Heart and Vessels (2024)
-
Fragmentation of Different Calcification Growth Patterns in Bicuspid Valves During Balloon Valvuloplasty Procedure
Annals of Biomedical Engineering (2023)
-
Left ventricular mass regression after aortic valve replacement with the TTK Chitra™ monoleaflet tilting disc valve
Indian Journal of Thoracic and Cardiovascular Surgery (2023)
-
Mid-term follow-up after aortic valve replacement with the Carpentier Edwards Magna Ease prosthesis
Journal of Cardiothoracic Surgery (2020)
-
Spatiotemporal Complexity of the Aortic Sinus Vortex as a Function of Leaflet Calcification
Annals of Biomedical Engineering (2019)