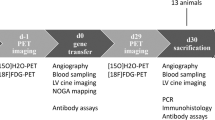
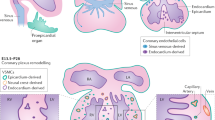
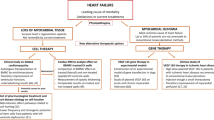
Abstract
Therapeutic induction of vascular growth may provide a treatment option for those patients with myocardial or peripheral ischemia who are not suited to conventional revascularization therapies. Some lymphatic vascular disorders may also be amenable to this therapy. However, clear evidence of efficacy must be obtained from phase 2 and 3 clinical trials before these new treatments can be entered into clinical practice. Apart from the clinical applications, gene transfer aimed at stimulating or blocking vascular growth with various growth factors, cytokines, transcription factors and receptors or their antagonists is useful for analyzing the effects of those molecules on the vasculature, especially when gene targeting results in lethality or when large animal models are required.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Debbie Maizels


Debbie Maizels

Similar content being viewed by others
References
Ylä-Herttuala, S. & Martin, J.F. Cardiovascular gene therapy. Lancet 355, 213–222 (2000).
Kootstra, N.A. & Verma, I.M. Gene therapy with viral vectors. Annu. Rev. Pharmacol. Toxicol. 43, 413–439 (2002).
Laitinen, M. et al. Gene transfer into the carotid artery using an adventitial collar. Comparison of the effectiveness of plasmid-liposome complexes, retroviruses, pseudotyped retroviruses and adenoviruses. Hum. Gene Ther. 8, 1645–1650 (1997).
Tripathy, S.K. et al. Long-term expression of erythropoietin in the systemic circulation of mice after intramuscular injection of a plasmid DNA vector. Proc. Natl. Acad. Sci. USA 93, 10876–10880 (1996).
Bergelson, J.M. et al. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275, 1320–1323 (1997).
Wickham, T.J. et al. Integrins αvβ3 and αvβ5 promote adenovirus internalisation but not virus attachment. Cell 73, 309–319 (1993).
Yang, Y. et al. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA 91, 4407–4411 (1994).
Lehrman, S. Virus treatment questioned after gene therapy death. Nature 401, 517–518 (1999).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996).
Trono, D. Lentiviral vectors: turning a deadly foe into a therapeutic agent. Gene Ther. 7, 20–23 (2000).
Svensson, E.C. et al. Efficient and stable transduction of cardiomyocytes after intramyocardial injection or intracoronary perfusion with recombinant adeno-associated virus vectors. Circulation 99, 201–205 (1999).
Monahan, P.E. & Samulski, R.J. AAV vectors: is clinical success on the horizon? Gene Ther. 7, 24–30 (2000).
Mesri, E.A., Federoff, H.J. & Brownlee, M. Expression of vascular endothelial growth factor from a defective herpes simplex virus type 1 amplicon vector induces angiogenesis in mice. Circ Res. 76, 161–167 (1995).
Fukumura, M. et al. Gene transfer to skeletal muscle and motor neurons by intramuscular injection of a novel minus strand RNA vector (Sendai virus vector). J. Gen. Med. 2 (suppl.), 24 (2000).
Airenne, K.J. et al. Baculovirus-mediated periadventitial gene transfer to rabbit carotid artery. Gene Ther. 7, 1499–1504 (2000).
Springer, M.L., Chen, A.S., Kraft, P.E., Bednarski, M. & Blau, H.M. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol. Cell. 2, 549–558 (1998).
Clackson, T. Regulated gene expression systems. Gene Ther. 7, 120–125 (2000).
Greco, O. et al. Novel chimeric gene promoters responsive to hypoxia and ionizing radiation. Gene Ther. 9, 1403–1411 (2002).
Koponen, J.K. et al. Doxycycline-regulated lentiviral vector system with a novel reverse transactivator rtTA2S-M2 shows a tight control of gene expression in vitro and in vivo. Gene Ther. 10, 459–466 (2003).
McManus, M.T. & Sharp, P.A. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 3, 737–747 (2002).
Marshall, E. Second child in French trial is found to have leukemia. Science 299, 320 (2003).
Laitinen, M. et al. Adenovirus-mediated gene transfer to lower limb artery of patients with chronic critical leg ischaemia. Hum. Gene Ther. 9, 1481–1486 (1998).
Arras, M. et al. The delivery of angiogenic factors to the heart by microsphere therapy. Nat. Biotechnol. 15, 159–162 (1998).
Villanueva, F.S. et al. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation 98, 1–5 (1998).
Ruoslahti, E. Specialization of tumour vasculature. Nat. Rev. Cancer 21, 83–90 (2002).
Alitalo, K. & Ferrara, N. Clinical applications of angiogenic growth factors and their inhibitors. Nat. Med. 5, 1359–1364 (1999).
Kärkkäinen, M.J. et al. A model for gene therapy of human hereditary lymphedema. Proc. Natl. Acad. Sci. USA 98, 12677–12682 (2001).
Szuba, A. et al. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 16, 1985–1987 (2002).
Saaristo, A., Karkkainen, M.J. & Alitalo, K. Insights into the molecular pathogenesis and targeted treatment of lymphedema. Ann. NY Acad. Sci. 979, 94–110 (2002).
Leung, D.W., Cachianes, G., Kuang, W.J., Goeddel, D.V. & Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989).
Dvorak, H.F., Brown, L.F., Detmar, M. & Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 146, 1029–1039 (1995).
Olofsson, B. et al. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc. Natl. Acad. Sci. USA. 93, 2576–2581 (1996).
Joukov, V. et al. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases EMBO J. 15, 290–298 (1996).
Achen, M.G. et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA. 95, 548–553 (1998).
Ogawa, S. et al. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J. Biol. Chem. 273, 31273–31282 (1998).
Park, J.E., Chen, H.H., Winer, J., Houck, K.A. & Ferrara, N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 269, 25646–25654 (1994).
Neufeld, G., Cohen, T., Gengrinovitch, S. & Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 13, 9–22 (1999).
Luttun, A. et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat. Med. 8, 831–840 (2002).
Hattori, K. et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1+ stem cells from bone-marrow microenvironment. Nat. Med. 8, 841–849 (2002).
Arras, M. et al. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J. Clin. Invest. 101, 40–50 (1998).
Rissanen, T.T. et al. Effects of VEGF-D gene transfer on vascular permeability and angiogenesis in rabbit skeletal muscle – comparison with other VEGFs. Circ. Res. (in the press).
Dor, Y. et al. Conditional switching of VEGF provides new insights into adult neovascularization and pro-angiogenic therapy. EMBO J. 21, 1939–1947 (2002).
Rissanen, T.T., Vajanto, I. & Ylä-Herttuala, S. Gene therapy for therapeutic angiogenesis in critically ischaemic lower limb – on the way to the clinic. Eur. J. Clin. Invest. 31, 651–666 (2001).
Saaristo, A. et al. Lymphangiogenic gene therapy with minimal blood vascular side effects. J. Exp. Med. 196, 719–730 (2002).
Yoon, Y. et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J. Clin. Invest. 111, 717–725 (2003).
Isner, J.M., Vale, P.R., Symes, J.F. & Losordo, D.W. Assessment of risks associated with cardiovascular gene therapy in human subjects. Circ. Res. 89, 389–400 (2001).
Davis, S. et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87, 1161–1169 (1996).
Maisonpierre, P.C. et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60 (1997).
Shyu, K.G., Manor, O., Magner, M., Yancopoulos, G.D. & Isner, J.M. Direct intramuscular injection of plasmid DNA encoding angiopoietin-1 but not angiopoietin-2 augments revascularization in the rabbit ischemic hindlimb. Circulation 98, 2081–2087 (1998).
Chae, J.K. et al. Coadministration of angiopoietin-1 and vascular endothelial growth factor enhances collateral vascularization. Arterioscler. Thromb. Vasc. Biol. 20, 2573–2578 (2000).
Arsic, N. et al. Induction of functional neovascularization by combined VEGF and angiopoietin-1 gene transfer using AAV vectors. Mol. Ther. 7, 450–459 (2003).
Javerzat, S., Auguste, P. & Bikfalvi, A. The role of fibroblast growth factors in vascular development. Trends Mol. Med. 10, 483–489 (2002).
Galzie, Z., Kinsella, A.R. & Smith, J.A. Fibroblast growth factors and their receptors. Biochem. Cell Biol. 75, 669–685 (1997).
Miller, D.L. et al. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2-null mice. Mol. Cell Biol. 20, 2260–2268 (2000).
Xu, X., Weinstein, M., Li, C. & Deng, C. Fibroblast growth factor receptors (FGFRs) and their roles in limb development. Cell Tissue Res. 296, 33–43 (1999).
Rissanen, T.T. et al. Fibroblast growth factor-4 induces vascular permeability, angiogenesis and arteriogenesis in a rabbit hindlimb ischemia model. FASEB J. 17, 100–102 (2003).
Kubo, H. et al. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc. Natl. Acad. Sci. USA 99, 8868–8873 (2002).
Giordano, F.J. et al. Intracoronary gene transfer of fibroblast growth factor-5 increases blood flow and contractile function in an ischaemic region of the heart. Nat. Med. 2, 534–539 (1996).
Simons, M. et al. Clinical trials in coronary angiogenesis: issues, problems, consensus. An expert panel summary. Circulation 102, e73–e86 (2000).
Morishita, R. et al. Therapeutic angiogenesis induced by human recombinant hepatocyte growth factor in rabbit hind limb ischemia model as cytokine supplement therapy. Hypertension 33, 1379–1384 (1999).
Ito, W.D. et al. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ. Res. 80, 829–837 (1997).
Takahashi, T. et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat. Med. 5, 434–438 (1999).
Cao, R. et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 9, 604–613 (2003).
Rissanen, T.T. et al. Expression of VEGF and VEGFR-2 (KDR/Flk-1) in ischemic skeletal muscle and its regeneration. Am. J. Pathol. 160, 1–11 (2002).
Barton-Davis, E.R., Shoturma, D.I., Musaro, A. Rosenthal, N. & Sweeney, H.L. Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc. Natl. Acad. Sci. USA 95, 15603–15607 (1998).
Penta, K. et al. Del1 induces integrin signaling and angiogenesis by ligation of αvβ3 . J. Biol. Chem. 274, 11101–11109 (1999).
Fataccioli, V. et al. Stimulation of angiogenesis by Cyr61 gene: A new therapeutic candidate. Hum. Gene Ther. 13, 1461–1470 (2002).
Dufourcq, P. et al. FrzA, a secreted frizzled related protein, induced angiogenic response. Circulation 106, 3097–3107 (2002).
Emanueli, C. et al. Nerve growth factor promotes angiogenesis and arteriogenesis in ischemic hindlimbs. Circulation 106, 2257–2262 (2002).
Emanueli, C. et al. Adenovirus-mediated human tissue kallikrein gene delivery induces angiogenesis in normoperfused skeletal muscle. Arterioscler. Thromb. Vasc. Biol. 20, 2379–2385 (2000).
Smith, R.S. Jr., Lin, K-F., Agata, J., Chao, L. & Chao, J. Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler. Thromb. Vasc. Biol. 22, 1279–1285 (2002).
Vincent, K.A. et al. Angiogenesis is induced in a rabbit model of hindlimb ischemia by naked DNA encoding an HIF-1α/VP16 hybrid transcription factor. Circulation 102, 2255–2261 (2000).
Bryant, M. et al. Tissue repair with a therapeutic transcription factor. Hum. Gene Ther. 11, 2143–2158 (2000).
Petrova, T.V. et al. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 21, 4593–4599 (2002).
Rebar, E.J. et al. Induction of angiogenesis in a mouse model using engineered transcription factors. Nat. Med. 8, 1427–1433 (2002).
Romano Di Peppe, S. et al. Adenovirus-mediated VEGF165 gene transfer enhances wound healing by promoting angiogenesis in CD1 diabetic mice. Gene Ther. 9, 1271–1277 (2002).
Deodato, B. et al. Recombinant AAV vector encoding human VEGF165 enhances wound healing. Gene Ther. 12, 777–785 (2002).
Rockson, S.G. Lymphedema. Am. J. Med. 110, 288–295 (2001).
Karkkainen, M.J. et al. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 25, 153–159 (2000).
Wright, M.J., Wightman, L.M., Latchman, D.S. & Marber, M.S. In vivo myocardial gene transfer: optimization and evaluation of intracoronary gene delivery in vivo. Gene Ther. 8, 1833–1839 (2001).
Vitadello, M., Schiaffino, M.V., Picard, A., Scarpa, M. & Schiaffino, S. Gene transfer in regenerating muscle. Hum. Gene Ther. 5, 11–18 (1994).
Schratzberger, P. et al. Reversal of experimental diabetic neuropathy by VEGF gene transfer. J. Clin. Invest. 107, 1083–1092 (2001).
Isner, J.M. et al. Clinical evidence of angiogenesis after arterial gene transfer of phVEGF165 in patient with ischaemic limb. Lancet 348, 370–374 (1996).
Baumgartner, I. et al. Constitutive expression of VEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation 97, 1114–1123 (1998).
Isner, J.M. et al. Treatment of thromboangiitis obliterans (Buerger's disease) by intramuscular gene transfer of vascular endothelial growth factor: preliminary clinical results. J. Vasc. Surg. 28, 964–973 (1998).
Losordo, D.W. et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischaemia. Circulation 98, 2800–2804 (1998).
Rosengart, T.K. et al. Angiogenesis gene therapy: phase I assessment of direct intramyocardial administration of an adenovirus vector expressing VEGF121 cDNA to individuals with clinically significant severe coronary artery disease. Circulation 100, 468–474 (1999).
Symes, J.F. et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann. Thorac. Surg. 68, 830–837 (1999).
Vale, P.R. et al. Left ventricular electromechanical mapping to assess efficacy of phVEGF165 gene transfer for therapeutic angiogenesis in chronic myocardial ischemia. Circulation 102, 965–974 (2000).
Laitinen, M. et al. Catheter-mediated vascular endothelial growth factor gene transfer to human coronary arteries after angioplasty. Hum. Gene Ther. 11, 263–270 (2000).
Vale, P.R. et al. Randomized, single-blind, placebo-controlled pilot study of catheter-based myocardial gene transfer for therapeutic angiogenesis using left ventricular electromechanical mapping of patients with chronic myocardial ischemia. Circulation 103, 2138–2143 (2001).
Rajagopalan, S., Shah, M., Luciano, A., Crystal, R. & Nabel, E.G. Adenovirus-mediated gene transfer of VEGF121 improves lower-extremity endothelial function and flow reserve. Circulation 104, 753–755 (2001).
Sarkar, N. et al. Effects of intramyocardial injection of phVEGF-A165 as sole therapy in patients with refractory coronary artery disease – 12-month follow-up: angiogenic gene therapy. J. Intern. Med. 250, 373–381 (2001).
Comerota, A.J. et al. Naked plasmid DNA encoding fibroblast growth factor type 1 for the treatment of end-stage unreconstructible lower extremity ischemia: preliminary results of a phase I trial. J. Vasc. Surg. 35, 930–936 (2002).
Losordo, D.W. et al. Phase 1/2 placebo-controlled, double-blind, dose-escalating trial of myocardial vascular endothelial growth factor 2 gene transfer by catheter delivery in patients with chronic myocardial ischemia. Circulation 105, 2012–2018 (2002).
Shyu, K.G., Chang, H., Wang, B.W. & Kuan, P. Intramuscular vascular endothelial growth factor gene therapy in patients with chronic critical leg ischemia. Am. J. Med. 114, 85–92 (2003).
Henry, T.D. et al. The VIVA trial. Vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 107, 1359–1365 (2003).
Simons, M. et al. Pharmacological treatment of coronary artery disease with recombinant fibroblast growth factor-2. Circulation 105, 788–793 (2002).
Lederman, R.J. et al. Therapeutic angiogenesis with recombinant fibroblast growth factor-2 for intermittent claudication (the TRAFFIC study): a randomised trial. Lancet 359, 2053–2058 (2002).
Seiler, C. et al. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease. Circulation 104, 2012–2017 (2001).
Grines, C.L. et al. Angiogenic Gene Therapy (AGENT) trial in patients with stable angina pectoris. Circulation 105, 1291–1297 (2002).
Mäkinen, K. et al. Increased vascularity detected by digital subtraction angiography after VEGF gene transfer to human lower limb artery. Mol. Ther. 6, 127–133 (2002).
Hedman, M. et al. Safety and feasibility of catheter-based local intracoronary VEGF gene transfer in the prevention of postangioplasty and in-stent restenosis and in the treatment of chronic myocardial ischemia. Circulation (in the press).
Stewart, D.J. et al. A phase 2, randomised, multicenter, 26-week study to assess the efficacy and safety of BIOBYPASS (AdGVVEGF121) delivered through minimally invasive surgery versus maximum medical treatment in patients with severe angina, advanced coronary artery disease, and no options for revascularizations. Circulation 106, 23–26 (2002).
Rajagopalan, S. et al. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease (RAVE). Late breaking clinical trials session. American College of Cardiology 2003, Chicago. J. Am. Coll. Cardiol. 41, 1604 (2003).
Kastrup, J. et al. Euroinject One trial. Late breaking clinical trials session. American College of Cardiology 2003, Chicago. J. Am. Coll. Cardiol. 41, 1603 (2003).
Pislaru, S., Janssens, S.P., Gersh, B.J. & Simari, R.D. Defining gene transfer before gene therapy. Circulation 106, 631–636 (2002).
Hiltunen, M.O. et al. Intravascular adenovirus-mediated VEGF-C gene transfer reduces neointima formation in balloon-denuded rabbit aorta. Circulation 102, 2262–2268 (2000).
Hiltunen, M.O. et al. Biodistribution of adenoviral vector to nontarget tissues after in in vivo gene transfer to arterial wall using intravascular and periadventitial gene delivery methods. FASEB J. 14, 2230–2236 (2000).
Pakkanen, T. et al. Periadventitial lacZ gene transfer to pig carotid arteries using a plasmid-liposome complexes. J. Gene Med. 2, 52–60 (2000).
Acknowledgements
Our studies were supported by the Finnish Academy of Sciences, the Finnish Technology Development Center, the European Union, the Novo Nordisk Foundation, the Sigrid Jusélius Foundation and the Ludwig Institute for Cancer Research. We thank M. Kärkkäinen, A. Saaristo, T. Rissanen, J. Rutanen and J. Markkanen for discussion and M. Poikolainen for help in preparing the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ylä-Herttuala, S., Alitalo, K. Gene transfer as a tool to induce therapeutic vascular growth. Nat Med 9, 694–701 (2003). https://doi.org/10.1038/nm0603-694
Issue Date:
DOI: https://doi.org/10.1038/nm0603-694
This article is cited by
-
Development of elastin-like polypeptide for targeted specific gene delivery in vivo
Journal of Nanobiotechnology (2020)
-
Recombinant baculovirus expressing the FrC-OVA protein induces protective antitumor immunity in an EG7-OVA mouse model
Journal of Biological Engineering (2019)
-
Correlative Imaging of the Murine Hind Limb Vasculature and Muscle Tissue by MicroCT and Light Microscopy
Scientific Reports (2017)
-
Angiogenic growth factors in myocardial infarction: a critical appraisal
Heart Failure Reviews (2017)
-
Vascular endothelial growth factor-A gene electrotransfer promotes angiogenesis in a porcine model of cardiac ischemia
Gene Therapy (2016)