Key Points
-
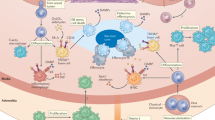
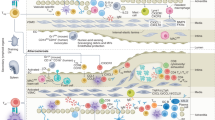
Atherosclerosis is an inflammatory disease of blood vessels. By causing myocardial infarction and stroke, it is a major cause of death globally.
-
Accumulation of cholesterol-containing plasma lipoproteins triggers inflammation in the artery wall, which can lead to atherosclerosis.
-
Monocyte-derived macrophages accumulate in the early atherosclerotic plaques.
-
Pattern-recognition receptors mediate cholesterol accumulation and inflammatory activation in plaque macrophages.
-
T cells enter plaques at an early stage and, importantly, contribute to plaque progression.
-
T-helper-1 cytokines (such as interferon-γ) and CD40 ligand are strongly pro-atherogenic, as they promote macrophage and endothelial-cell activation, platelet aggregation and thrombosis
-
Regulatory T cells producing immunomodulatory cytokines (such as transforming growth factor-β and interleukin-10) reduce the progression of atherosclerosis.
-
Systemic humoral immunity to oxidized lipoproteins also inhibits disease progression.
-
The long silent phase of atherosclerosis is characterized by smouldering inflammation in plaques. Activation of this pro-inflammatory process can cause plaque activation, rupture and thrombosis. This leads to clinical syndromes such as myocardial infarction and stroke.
Abstract
Immune responses participate in every phase of atherosclerosis. There is increasing evidence that both adaptive and innate immunity tightly regulate atherogenesis. Although improved treatment of hyperlipidaemia reduces the risk for cardiac and cerebral complications of atherosclerosis, these remain among the most prevalent of diseases and will probably become the most common cause of death globally within 15 years. This Review focuses on the role of immune mechanisms in the formation and activation of atherosclerotic plaques, and also includes a discussion of the use of inflammatory markers for predicting cardiovascular events. We also outline possible future targets for prevention, diagnosis and treatment of atherosclerosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Murray, C. J. & Lopez, A. D. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet 349, 1436–1442 (1997).
Jonasson, L., Holm, J., Skalli, O., Bondjers, G. & Hansson, G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6, 131–138 (1986).
Bobryshev, Y. V. & Lord, R. S. A. S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovas. Res. 29, 689–696 (1995).
Kovanen, P. T., Kaartinen, M. & Paavonen, T. Infiltrates of activated mast cells at the site of coronary atheromatous erosion or rupture in myocardial infarction. Circulation 92, 1084–1088 (1995).
Jonasson, L., Holm, J., Skalli, O., Gabbiani, G. & Hansson, G. K. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J. Clin. Invest. 76, 125–131 (1985).
Plump, A. S. et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343–353 (1992).
Piedrahita, J. A., Zhang, S. H., Hagaman, J. R., Oliver, P. M. & Maeda, N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl Acad. Sci. USA 89, 4471–4475 (1992).
Ishibashi, S., Goldstein, J. L., Brown, M. S., Herz, J. & Burns, D. K. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J. Clin. Invest. 93, 1885–1893 (1994).
Cybulsky, M. I. & Gimbrone, M. A. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherosclerosis. Science 251, 788–791 (1991).
Nakashima, Y., Raines, E. W., Plump, A. S., Breslow, J. L. & Ross, R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the apoE-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 18, 842–851 (1998).
Dai, G. et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and-resistant regions of human vasculature. Proc. Natl Acad. Sci. USA 101, 14871–14876 (2004).
Rajavashisth, T. B. et al. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature 344, 254–257 (1990).
Smith, J. D. et al. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl Acad. Sci. USA 92, 8264–8268 (1995).
Li, H., Cybulsky, M. I., Gimbrone, M. A. & Libby, P. Inducible expression of vascular cell adhesion molecule-1 by vascular smooth muscle cells in vitro and within rabbit atheroma. Am. J. Pathol. 143, 1551–1559 (1993).
Dong, Z. M. et al. The combined role of P- and E-selectins in atherosclerosis. J. Clin. Invest. 102, 145–152 (1998).
Cybulsky, M. I. et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J. Clin. Invest. 107, 1255–1262 (2001).
Boring, L., Gosling, J., Cleary, M. & Charo, I. F. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394, 894–897 (1998).
Gu, L. et al. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 2, 275–281 (1998).
Mach, F. et al. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J. Clin. Invest. 104, 1041–1050 (1999).
Haley, K. J. et al. Overexpression of eotaxin and the CCR3 receptor in human atherosclerosis: using genomic technology to identify a potential novel pathway of vascular inflammation. Circulation 102, 2185–2189 (2000).
Minami, M. et al. Expression of SR-PSOX, a novel cell-surface scavenger receptor for phosphatidylserine and oxidized LDL in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 21, 1796–1800 (2001).
Veillard, N. R. et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ. Res. 94, 253–261 (2004).
Combadiere, C. et al. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 107, 1009–1016 (2003).
Lesnik, P., Haskell, C. A. & Charo, I. F. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J. Clin. Invest. 111, 333–340 (2003).
Steinberg, D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 272, 20963–20966 (1997).
Peiser, L., Mukhopadhyay, S. & Gordon, S. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 14, 123–128 (2002).
Nicoletti, A. et al. The macrophage scavenger receptor type A directs modified proteins to antigen presentation. Eur. J. Immunol. 29, 512–521 (1999).
Moore, K. J. et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Invest. 115, 2192–2201 (2005).
Bodzioch, M. et al. The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease. Nature Genet. 22, 347–351 (1999).
Janeway, C. A. Jr & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Edfeldt, K., Swedenborg, J., Hansson, G. K. & Yan, Z. Q. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation 105, 1158–1161 (2002).
Kol, A., Lichtman, A. H., Finberg, R. W., Libby, P. & Kurt-Jones, E. A. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J. Immunol. 164, 13–17 (2000).
Xu, X. H. et al. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation 104, 3103–3108 (2001).
Miller, Y. I. et al. Minimally modified LDL binds to CD14, induces macrophage spreading via TLR4/MD-2, and inhibits phagocytosis of apoptotic cells. J. Biol. Chem. 278, 1561–1568 (2003).
Michelsen, K. S. et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl Acad. Sci. USA 101, 10679–10684 (2004).
Bjorkbacka, H. et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nature Med. 10, 416–421 (2004).
Paulsson, G., Zhou, X., Tö rnquist, E. & Hansson, G. K. Oligoclonal T cell expansions in atherosclerotic lesions of apoE-deficient mice. Arterioscler. Thromb. Vasc. Biol. 20, 10–17 (2000).
Stemme, S., Holm, J. & Hansson, G. K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler. Thromb. 12, 206–211 (1992).
Angeli, V. et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity 21, 561–574 (2004).
Wang, X. et al. Positional identification of TNFSF4, encoding OX40 ligand, as a gene that influences atherosclerosis susceptibility. Nature Genet. 37, 365–372 (2005).
Swanberg, M. et al. MHC2TA is associated with differential MHC molecule expression and susceptibility to rheumatoid arthritis, multiple sclerosis and myocardial infarction. Nature Genet. 37, 486–494 (2005).
Stemme, S. et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc. Natl Acad. Sci. USA 92, 3893–3897 (1995).
de Boer, O. J. et al. Unstable atherosclerotic plaques contain T-cells that respond to Chlamydia pneumoniae. Cardiovasc. Res. 48, 402–408 (2000).
Palinski, W. et al. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl Acad. Sci. USA 86, 1372–1376 (1989).
Palinski, W. et al. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J. Clin. Invest. 98, 800–814 (1996).
Chicz, R. M. et al. Specificity and promiscuity among naturally processed peptides bound to HLA-DR alleles. J. Exp. Med. 178, 27–47 (1993).
Zhou, X., Caligiuri, G., Hamsten, A., Lefvert, A. K. & Hansson, G. K. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 21, 108–114 (2001).
Stemme, S. et al. T lymphocytes from human atherosclerotic plaques recognize oxidized LDL. Proc. Natl Acad. Sci. USA 92, 3893–3897 (1995).
Shaw, P. X. et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J. Clin. Invest. 105, 1731–17340 (2000).
Binder, C. J. et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nature Med. 9, 736–743 (2003).
Zhou, X., Nicoletti, A., Elhage, R. & Hansson, G. K. Transfer of CD4+ T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation 102, 2919–2922 (2000).
Tupin, E. et al. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J. Exp. Med. 199, 417–422 (2004).
Melian, A., Geng, Y. J., Sukhova, G. K., Libby, P. & Porcelli, S. A. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am. J. Pathol. 155, 775–786 (1999).
Nakai, Y. et al. Natural killer T cells accelerate atherogenesis in mice. Blood 104, 2051–2059 (2004).
Uyemura, K. et al. Cross-regulatory roles of interleukin (IL)-12 and IL-10 in atherosclerosis. J. Clin. Invest. 97, 2130–2138 (1996).
Frostegard, J. et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis 145, 33–43. (1999).
Gupta, S. et al. IFN-γ potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 99, 2752–2761 (1997).
Buono, C. et al. Influence of interferon-γ on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler. Thromb. Vasc. Biol. 23, 454–460 (2003).
Davenport, P. & Tipping, P. G. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 163, 1117–1125 (2003).
Elhage, R. et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc. Res. 59, 234–240 (2003).
Branen, L. et al. Inhibition of tumor necrosis factor-α reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler. Thromb. Vasc. Biol. 24, 2137–2142 (2004).
Buono, C. et al. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc. Natl Acad. Sci. USA 102, 1596–1601 (2005).
Whitman, S. C., Ravisankar, P., Elam, H. & Daugherty, A. Exogenous interferon-γ enhances atherosclerosis in apolipoprotein E−/− mice. Am. J. Pathol. 157, 1819–1824 (2000).
Laurat, E. et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 104, 197–202 (2001).
Paigen, B., Morrow, A., Brandon, C., Mitchell, D. & Holmes, P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis 57, 65–73 (1985).
Huber, S. A., Sakkinen, P., David, C., Newell, M. K. & Tracy, R. P. T helper-cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation 103, 2610–2616 (2001).
Mallat, Z. et al. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ. Res. 89, E41–E45 (2001).
Whitman, S. C., Ravisankar, P. & Daugherty, A. Interleukin-18 enhances atherosclerosis in apolipoprotein E−/− mice through release of interferon-γ. Circ. Res. 90, e34–e38 (2002).
King, V. L., Szilvassy, S. J. & Daugherty, A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor−/− mice. Arterioscler. Thromb. Vasc. Biol. 22, 456–461 (2002).
Shimizu, K., Shichiri, M., Libby, P., Lee, R. T. & Mitchell, R. N. Th2-predominant inflammation and blockade of IFN-γ signaling induce aneurysms in allografted aortas. J. Clin. Invest. 114, 300–308 (2004).
Friesel, R., Komoriya, A. & Maciag, T. Inhibition of endothelial cell proliferation by γ-interferon. J. Cell Biol. 104, 689–696 (1987).
Hansson, G. K., Hellstrand, M., Rymo, L., Rubbia, L. & Gabbiani, G. Interferon γ inhibits both proliferation and expression of differentiation-specific α-smooth muscle actin in arterial smooth muscle cells. J. Exp. Med. 170, 1595–1608 (1989).
Amento, E. P., Ehsani, N., Palmer, H. & Libby, P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler. Thromb. 11, 1223–1230 (1991).
van Hinsbergh, V. W. M., van den Berg, E. A., Fiers, W. & Dooijewaard, G. Tumor necrosis factor induces the production of urokinase-type plasminogen activator by human endothelial cells. Blood 75, 1991–1998 (1990).
Lee, E. et al. Regulation of matrix metalloproteinases and plasminogen activator inhibitor-1 synthesis by plasminogen in cultured human vascular smooth muscle cells. Circ. Res. 78, 44–49 (1996).
Saren, P., Welgus, H. G. & Kovanen, P. T. TNF-α and IL-1β selectively induce expression of 92-kDa gelatinase by human macrophages. J. Immunol. 157, 4159–4165 (1996).
Jovinge, S. et al. Evidence for a role of tumor necrosis factor α in disturbances of triglyceride and glucose metabolism predisposing to coronary heart disease. Metabolism 47, 113–118 (1998).
Boquist, S. et al. Alimentary lipemia, postprandial triglyceride-rich lipoproteins, and common carotid intima-media thickness in healthy, middle-aged men. Circulation 100, 723–728 (1999).
Beutler, B. & Cerami, A. Cachectin and tumour necrosis factor as two sided of the same biological coin. Nature 320, 584–588 (1986).
Mach, F., Schoenbeck, U., Bonnefoy, J.-Y., Pober, J. & Libby, P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of CD40. Induction of collagenase, stromelysin, and tissue factor. Circulation 96, 396–399 (1997).
Mach, F. et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages- implications for CD40–CD40 ligand signaling in atherosclerosis. Proc. Natl Acad. Sci. USA 94, 1931–1936 (1997).
Henn, V. et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391, 591–594 (1998).
Mach, F., Schö nbeck, U., Sukhova, G. K., Atkinson, E. & Libby, P. Reduction of atherosclerosis in mice by inhibition of CD40 signalling. Nature 394, 200–203 (1998).
Lutgens, E. et al. Requirement for CD154 in the progression of atherosclerosis. Nature Med. 5, 1313–1316 (1999).
Mallat, Z. et al. Protective role of interleukin-10 in atherosclerosis. Circ. Res. 85, e17–e24 (1999).
Pinderski Oslund, L. J. et al. Interleukin-10 blocks atherosclerotic events in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 19, 2847–53 (1999).
Caligiuri, G. et al. Interleukin-10 deficiency increases atherosclerosis, thrombosis, and low-density lipoproteins in apolipoprotein E knockout mice. Mol. Med. 9, 10–17 (2003).
Grainger, D. J. et al. The serum concentration of active transforming growth factor-β is severely depressed in advanced atherosclerosis. Nature Med. 1, 74–79 (1995).
Mallat, Z. et al. Inhibition of transforming growth factor-β signaling accelerates atherosclerosis and induces an unstable plaque phenotype in mice. Circ. Res. 89, 930–934 (2001).
Lutgens, E. et al. Transforming growth factor-β mediates balance between inflammation and fibrosis during plaque progression. Arterioscler. Thromb. Vasc. Biol. 22, 975–982 (2002).
Robertson, A. K. et al. Disruption of TGF-β signaling in T cells accelerates atherosclerosis. J. Clin. Invest. 112, 1342–1350 (2003).
Gojova, A. et al. Specific abrogation of transforming growth factor-β signaling in T cells alters atherosclerotic lesion size and composition in mice. Blood 102, 4052–4058 (2003).
Ait-Oufella, H. et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nature Med. 12, 178–180 (2006).
Salonen, J. T. et al. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet 339, 883–887 (1992).
Fredrikson, G. N. et al. Identification of immune responses against aldehyde-modified peptide sequences in apoB associated with cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 23, 872–878 (2003).
Shaw, P. X. et al. Human-derived anti-oxidized LDL autoantibody blocks uptake of oxidized LDL by macrophages and localizes to atherosclerotic lesions in vivo. Arterioscler. Thromb. Vasc. Biol. 21, 1333–1339 (2001).
Kol, A., Sukhova, G. K., Lichtman, A. H. & Libby, P. Chlamydial heat shock protein 60 localizes in human atheroma and regulates macrophage tumor necrosis factor-α and matrix metalloproteinase expression. Circulation 98, 300–307 (1998).
Xu, Q. et al. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet 341, 255–259 (1993).
Perschinka, H. et al. Cross-reactive B-cell epitopes of microbial and human heat shock protein 60/65 in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23, 1060–1065 (2003).
Caligiuri, G., Nicoletti, A., Poirier, B. & Hansson, G. K. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J. Clin. Invest. 109, 745–753 (2002).
Libby, P. & Aikawa, M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nature Med. 8, 1257–1262 (2002).
Henney, A. M. et al. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc. Natl Acad. Sci. USA 88, 8154–8158 (1991).
Galis, Z. S., Sukhova, G. K., Lark, M. W. & Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnarable regions of human atherosclerotic plaques. J. Clin. Invest. 94, 2493–2503 (1994).
Dollery, C. & Libby, P. Atherosclerosis and proteinase activation. Cardiovasc. Res. 69, 625–635 (2006).
Buchner, K. et al. CD40 ligand is selectively expressed on CD4+ T cells and platelets: implications for CD40–CD40L signalling in atherosclerosis. J. Pathol. 201, 288–295 (2003).
Poon, M., Badimon, J. J. & Fuster, V. Overcoming restenosis with sirolimus: from alphabet soup to clinical reality. Lancet 359, 619–622 (2002).
Jonasson, L., Holm, J. & Hansson, G. K. Cyclosporin A inhibits smooth muscle proliferation in the vascular response to injury. Proc. Natl Acad. Sci. USA 85, 2303–2306 (1988).
Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344, 1383–1389 (1994).
Ridker, P. M. et al. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 352, 20–28 (2005).
Greenwood, J., Steinman, L. & Zamvil, S.S. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nature Rev. Immunol. 6, 358–370
Takemoto, M. & Liao, J. K. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler. Thromb. Vasc. Biol. 21, 1712–1719 (2001).
Kwak, B., Mulhaupt, F., Myit, S. & Mach, F. Statins as a newly recognized type of immunomodulator. Nature Med. 6, 1399–1402 (2000).
Mehra, M. R. & Raval, N. Y. Metaanalysis of statins and survival in de novo cardiac transplantation. Transplant. Proc. 36, 1539–1541 (2004).
McCarey, D. W. et al. Trial of atorvastatin in rheumatoid arthritis (TARA): double-blind, randomised placebo-controlled trial. Lancet 363, 2015–2021 (2004).
Youssef, S. et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 420, 78–84 (2002).
Marx, N. et al. PPAR activators as antiinflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ. Res. 90, 703–710 (2002).
Staels, B. et al. Activation of human aortic smooth-muscle cells is inhibited by PPARα but not by PPARγ activators. Nature 393, 790–793 (1998).
Fitzgerald, G. A. Coxibs and cardiovascular disease. N. Engl. J. Med. 351, 1709–1711 (2004).
Solomon, S. D. et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 352, 1071–1080 (2005).
Mattner, J. et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434, 525–529 (2005).
Liuzzo, G. et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N. Engl. J. Med. 331, 417–424 (1994).
Ridker, P. M., Hennekens, C. H., Buring, J. E. & Rifai, N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 342, 836–843 (2000).
Danesh, J. et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N. Engl. J. Med. 350, 1387–1397 (2004).
Palinski, W., Miller, E. & Witztum, J. L. Immunization of low density lipoprotein (LDL) receptor-deficient rabbits with homologous malondialdehyde-modified LDL reduces atherogenesis. Proc. Natl Acad. Sci. USA 92, 821–825 (1995).
Ameli, S. et al. Effect of immunization with homologous LDL and oxidized LDL on early atherosclerosis in hypercholesterolemic rabbits. Arterioscler. Thromb. Vasc. Biol. 16, 1074–1079 (1996).
George, J. et al. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclerosis 138, 147–152 (1998).
Freigang, S., Hörkkö, S., Miller, E., Witztum, J. L. & Palinski, W. Immunization of LDL receptor-deficient mice with homologous malondialdehyde-modified and native LDL reduces progression of atherosclerosis by mechanisms other than induction of high titers of antibodies to oxidative neoepitopes. Arterioscler. Thromb. Vasc. Biol. 18, 1972–1982 (1998).
Fredrikson, G. N. et al. Inhibition of atherosclerosis in apoE-null mice by immunization with apoB-100 peptide sequences. Arterioscler. Thromb. Vasc. Biol. 23, 879–884 (2003).
Zhou, X., Robertson, A. K., Hjerpe, C. & Hansson, G. K. Adoptive transfer of CD4+ T cells reactive to modified low-density lipoprotein aggravates atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 26, 864–870 (2006).
Afek, A. et al. Immunization of low-density lipoprotein receptor deficient (LDL-RD) mice with heat shock protein 65 (HSP-65) promotes early atherosclerosis. J. Autoimmun. 14, 115–121 (2000).
Harats, D., Yacov, N., Gilburd, B., Shoenfeld, Y. & George, J. Oral tolerance with heat shock protein 65 attenuates Mycobacterium tuberculosis-induced and high-fat-diet-driven atherosclerotic lesions. J. Am. Coll. Cardiol. 40, 1333–1338 (2002).
Maron, R. et al. Mucosal administration of heat shock protein-65 decreases atherosclerosis and inflammation in aortic arch of low-density lipoprotein receptor-deficient mice. Circulation 106, 1708–1715 (2002).
George, J. et al. Induction of early atherosclerosis in LDL-receptor-deficient mice immunized with β2-glycoprotein I. Circulation 98, 1108–1115 (1998).
Acknowledgements
We regret that we have not been able to cite many important papers owing to space limitations. Our research is supported by grants from the Swedish Research Council, Heart-Lung Foundation, European Community, US National Institutes of Health and Leducq Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Plaque
-
An atherosclerotic lesion consisting of a fibrotic cap surrounding a lipid-rich core. The lesion is the site of inflammation, lipid accumulation and cell death. Also known as an atheroma.
- Myocardial infarction
-
An episode of acute cardiac ischaemia that leads to death of heart muscle cells. It is usually caused by a thrombotic atherosclerotic plaque.
- Ischaemic stroke
-
An episode of acute regional ischaemia in the brain leading to nerve-cell death. It is usually caused by thrombi or emboli from atherosclerotic plaques.
- Aneurysm
-
The local dilatation of an artery caused by weakening of the artery wall. Some, but not all, aneurysms are caused by atherosclerosis.
- Intima
-
The innermost layer of an artery, which consists of loose connective tissue and is covered by a monolayer of endothelium. Atherosclerotic plaques form in the intima.
- Fibrous cap
-
A structure composed of a dense collagen-rich extracellular matrix with occasional smooth muscle cells, macrophages and T cells that typically overlies the characteristic central lipid core of plaques.
- Scavenger receptors
-
Cell-membrane proteins that take up oxidatively or otherwise modified low-density lipoproteins.
- Vasa vasorum
-
Small nutrient vessels in the normal adventitia and outer media of the artery wall, which can also give rise to microvessels in the plaque.
- Tissue factor
-
A procoagulant that stimulates thrombus formation, when in contact with blood, by accelerating the action of factors VIIa and Xa.
- Angina pectoris
-
A reversible attack of chest discomfort, usually caused by an imbalance between the oxygen demand of the working heart muscle and the insufficient supply through narrow, atherosclerotic coronary arteries.
- Angioplasty
-
A percutaneous catheter procedure that inflates a balloon in areas of narrowing (stenosis) in arteries.
- Statins
-
A class of drugs that inhibit the rate-limiting enzyme (3-hydroxy-3-methylglutaryl coenzyme A reductase) in the pathway of cholesterol biosynthesis.
- Peroxisome-proliferator-activated receptors
-
Nuclear receptors that participate in the regulation of cellular metabolism and differentiation.
- Thiazolidinedione
-
A class of medication, used to treat diabetes, that binds peroxisome-proliferator-activated receptor-γ.
- C-reactive protein
-
An acute-phase reactant protein, the plasma concentration of which increases in inflammatory states.
Rights and permissions
About this article
Cite this article
Hansson, G., Libby, P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol 6, 508–519 (2006). https://doi.org/10.1038/nri1882
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1882
This article is cited by
-
Shared inflammatory pathways of rheumatoid arthritis and atherosclerotic cardiovascular disease
Nature Reviews Rheumatology (2023)
-
A Lipid-Structured Model of Atherosclerotic Plaque Macrophages with Lipid-Dependent Kinetics
Bulletin of Mathematical Biology (2023)
-
Mesenchymal stem cell transplantation alleviated atherosclerosis in systemic lupus erythematosus through reducing MDSCs
Stem Cell Research & Therapy (2022)
-
C/EBPβ/AEP signaling couples atherosclerosis to the pathogenesis of Alzheimer’s disease
Molecular Psychiatry (2022)
-
Tissue-engineered collagenous fibrous cap models to systematically elucidate atherosclerotic plaque rupture
Scientific Reports (2022)