-
PDF
- Split View
-
Views
-
Cite
Cite
Reinhard C. Funck, Jean-Jacques Blanc, Hans-Helge Mueller, Carmen Schade-Brittinger, Christophe Bailleul, Bernhard Maisch, for the BioPace Study Group, Biventricular stimulation to prevent cardiac desynchronization: rationale, design, and endpoints of the ‘Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BioPace)’ study, EP Europace, Volume 8, Issue 8, August 2006, Pages 629–635, https://doi.org/10.1093/europace/eul075
Close - Share Icon Share
Abstract
Despite the deleterious effects of cardiac dyssynchrony and the positive effects of cardiac resynchronization therapy, patients with high-degree atrioventricular block continue to receive desynchronizing right ventricular (RV) pacing systems. Although it is unclear whether the negative effects of RV pacing and left bundle branch block (LBBB) are comparable, and whether they depend on the presence and the degree of structural heart disease, one may hypothesize that RV pacing may have similar effects to LBBB. In the BioPace trial, the long-term effects of RV pacing vs. biventricular pacing will be prospectively compared in 1200 pacemaker patients with high likelihood of mostly paced ventricular events, regardless of whether in sinus rhythm or in atrial fibrillation (AF). After echocardiographic examination of left ventricular (LV) function, patients will be randomly assigned to the implantation of an RV vs. a biventricular pacing system and followed for up to 5 years. Primary study endpoints are survival, quality of life (QoL), and the distance covered in a 6-min hall walk (6-MHW) at 24 months after implantation. Secondary endpoints are QoL and the 6-MHW result at 12 months after implantation, hospitalization rate, LV dimensions, LV ejection fraction, and the development of chronic AF and other adverse events.
Introduction
Cardiac dyssynchrony has deleterious effects on cardiac function by depressing left ventricular (LV) mechanical performance while increasing myocardial oxygen consumption.1 In addition, it probably causes LV remodelling.2,3 Therefore, cardiac dyssynchrony accelerates the progression of chronic congestive heart failure (CHF), and studies in patients with CHF and left bundle branch block (LBBB) have demonstrated its negative prognostic impact on survival.4 Cardiac resynchronization therapy (CRT) has shown that (i) LV dilatation and dysfunction in the presence of cardiac asynchrony are potentially reversible and (ii) most of the positive electromechanical effects of CRT can, at least in the short-term, be achieved by stimulating the left ventricle alone, confirming the importance of direct LV pacing.
Although cardiac dyssynchrony is usually associated with spontaneous LBBB, it may also be an ‘iatrogenic’ adverse effect of right ventricular (RV) pacing. Furthermore, the desynchronizing effects of RV pacing appear similar to those observed with LBBB.1 However, the prognostic implications of RV pacing-induced cardiac desynchronization in patients with and without LV dysfunction are largely unclear. In addition, the conditions in which long-term RV pacing does not induce cardiac remodelling despite obvious electrical desynchronization are not known. We, therefore, designed the Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BioPace) trial to study prospectively the long-term effects of biventricular vs. conventional RV pacing in patients with an indication for permanent ventricular pacing and any LV size and function.
Background
Ventricular pacing is the only mode of prevention of profound bradycardia or asystole in patients with complete atrioventricular (AV) block. Conventional RV pacing, however, is associated with abnormal ventricular activation, causing a prominent electromechanical delay in patients with spontaneously narrow QRS complexes.5 The main adverse consequences of abnormal ventricular activation are the depression of systolic function (dp/dt) and cardiac output and an increase in filling pressures.6 These observations were not limited to animal experiments7–9 or adult humans with pre-existent LV dysfunction, but were even noted in infants without structural heart disease.10 The duration of the stimulated QRS appears to be a major determinant of the degree of LV dysfunction induced by RV pacing, as observed by Schwaab et al.11 in a radionuclide ventriculographic study, and by Tse et al.,12 who found a significantly longer stimulated QRS duration with RV apical than with RV outflow tract (OT) pacing. At 18 months after the onset of RV pacing, patients paced at the RVOT had a significantly higher LV ejection fraction (EF) than patients paced at the RV apex, whose LVEF had decreased.12 As shown by Heyndrickx et al.,13 cardiac asynchrony also induces abnormal relaxation, which in turn could explain myocardial perfusion defects and thus regional wall motion abnormalities. Regional wall motion abnormalities were more frequently observed in patients paced at the RV apex compared with those at RVOT pacing. It is noteworthy that these differences were detectable at 18, but not at 6 months after the onset of RV pacing.14 However, these beneficial mechanistic effects of RVOT pacing do not necessarily translate into a better clinical outcome. This could, at least, not be shown in a rather short-term crossover study.15
There is currently no evidence that cardiac desynchronization by RV pacing is less adverse than spontaneous LBBB, which is usually associated with structural heart disease.4,16 In patients with LV dilatation and dysfunction, the prognostic content of spontaneous LBBB and RV pacing-induced QRS widening are very likely to be similar.17 This argument is supported by the results of the DAVID study, which showed a higher event-free survival in recipients of implantable cardioverter defibrillators (ICDs) programmed with a VVI backup pacing rate of 40 bpm than in patients whose ICD had been programmed to DDD pacing, such that the RV was nearly incessantly paced.2 This is concordant with the results of other trials where patients paced for AV block at the ventricular level, particularly in the presence of atrial fibrillation (AF), had a less favourable prognosis than patients with sick sinus syndrome.18 Furthermore, in the PAVE study, biventricular pacing was associated with a significantly greater functional capacity than RV pacing after AV nodal ablation for chronic AF.19
Rationale for direct biventricular pacing in AV block
Alternatives proposed to mitigate the negative effects of RV apical pacing have been RVOT, RV septal, dual-site RV, and His-bundle pacing. With RV septal pacing, the QRS duration was reduced in 64% of patients and a negative correlation between the duration of the paced QRS complex and LVEF was observed. However, the method may be time-consuming and RV septal pacing is not predictably associated with a shorter-paced QRS duration than RV apical pacing.11 Regardless of QRS duration, LV stimulation was found to be superior to RV stimulation from the standpoint of LV mechanical performance.20 While the QRS complex was shorter during biventricular pacing, the main haemodynamic benefit seemed attributable to LV pacing. Thus, from an electromechanical point of view, LV-based pacing is probably superior to any RV pacing configuration and should be preferred to dual-site RV pacing. A limitation of His bundle pacing is its inapplicability in patients with infra-Hisian conduction abnormalities, which are common in the clinical setting of CHF.4 This study has, therefore, been designed to compare biventricular with conventional RV pacing. LV pacing only was deliberately not chosen because of the persistent risk of LV lead dislodgement in a potentially pacemaker-dependent patient population.
Rationale for the use of ICDs as pacing devices
In its initial phase, the BioPace study has started without the possibility to implant ICDs. However, meanwhile it became more and more accepted that patients with EFs≤35% represent a high-risk population for sudden cardiac death (SCD), even if prior arrhythmic events are lacking, and that ICDs can considerably reduce their risk. This was proven in two major prospective randomized trials, the Multicenter Automatic Defibrillator Implantation Trial (MADIT II)12 and the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) study.21
In the MADIT II study, the effect of ICD therapy on SCD reduction in patients with history of myocardial infarction and LVEFs≤30% was investigated. The ICD reduced the risk of SCD by 28%. The SCD-HeFT study included heart failure patients [New York Heart Association (NYHA) class II–III] with ischaemic and non-ischaemic heart disease and slightly less impaired LV function (EF≤35%). The relative risk reduction achieved by the ICD was 23% compared with placebo therapy in this group of patients. These important results on primary SCD prevention could not be ignored by a large study such as BioPace. Therefore, it was only a logical consequence of these published results and a reaction to impending guideline modification22 to add an amendment to the BioPace study protocol so that the patients who fulfil the BioPace inclusion criteria and who, in terms of LV dysfunction, meet the SCD-HeFT and/or the MADIT II criteria can be included in the BioPace study and be implanted with ICDs to be used primarily as pacing devices. This amendment was made in August 2005. It did not alter the inclusion criteria of the study but merely reduced the exclusion criteria. The decision to implant an ICD instead of a pacemaker will only be based on the insight that patients with severely impaired LVEFs are prone to SCD. No other accepted tachyarrhythmia indication for the implantation of an ICD may be present. The decision whether a patient will be scheduled for the implantion of an ICD or a pacemaker system will be left to the discretion of the physician. From the biometrician's point of view, the extension of eligibility criteria will increase the external validity of the trial.
Main objectives of the study
The primary objective of the study is to determine whether synchronous biventricular pacing to prevent iatrogenic ventricular desynchronization confers a clinical benefit in patients with conventional indications for permanent ventricular pacing, regardless of spontaneous QRS duration and morphology or LV size and function. Clinical benefit is defined as a higher survival rate, longer distance covered during the 6-min hall walk (6-MHW) test, and better quality of life (QoL), ascertained by the Minnesota Living with Heart Failure Questionnaire (Table 1). Cardiac structure and function will be investigated by echocardiography.
Study endpoints
| Primary |
| Survival after randomization |
| Functional capacity measured by the distance covered in the 6-MHW test at 24 months |
| QoL measured by the Minnesota Living With Heart Failure questionnaire at 24 months |
| Secondary |
| Functional capacity measured by the distance covered in the 6-MHW test at 12 months |
| QoL measured by the Minnesota Living With Heart Failure questionnaire at 12 months |
| Rate and duration of hospitalization for progression of CHF |
| Rates and lengths of hospitalizations for management of adverse cardiovascular events |
| Rates and lengths of hospitalizations for any reason |
| Cardiac structure and function by echocardiographic examination including |
| LV end-diastolic and endsystolic diameters |
| LVEF |
| Left atrial dimensions |
| Degree of mitral and tricuspid regurgitation |
| Pulmonary artery systolic pressure |
| Intra- and interventricular mechanical delays |
| Rates of adverse events related to |
| LV lead |
| All leads |
| Success rate of the St Jude Medical LV lead implantation |
| Incidence of permanent AF, defined as the presence of AF in two consecutive ECGs |
| Primary |
| Survival after randomization |
| Functional capacity measured by the distance covered in the 6-MHW test at 24 months |
| QoL measured by the Minnesota Living With Heart Failure questionnaire at 24 months |
| Secondary |
| Functional capacity measured by the distance covered in the 6-MHW test at 12 months |
| QoL measured by the Minnesota Living With Heart Failure questionnaire at 12 months |
| Rate and duration of hospitalization for progression of CHF |
| Rates and lengths of hospitalizations for management of adverse cardiovascular events |
| Rates and lengths of hospitalizations for any reason |
| Cardiac structure and function by echocardiographic examination including |
| LV end-diastolic and endsystolic diameters |
| LVEF |
| Left atrial dimensions |
| Degree of mitral and tricuspid regurgitation |
| Pulmonary artery systolic pressure |
| Intra- and interventricular mechanical delays |
| Rates of adverse events related to |
| LV lead |
| All leads |
| Success rate of the St Jude Medical LV lead implantation |
| Incidence of permanent AF, defined as the presence of AF in two consecutive ECGs |
Study endpoints
| Primary |
| Survival after randomization |
| Functional capacity measured by the distance covered in the 6-MHW test at 24 months |
| QoL measured by the Minnesota Living With Heart Failure questionnaire at 24 months |
| Secondary |
| Functional capacity measured by the distance covered in the 6-MHW test at 12 months |
| QoL measured by the Minnesota Living With Heart Failure questionnaire at 12 months |
| Rate and duration of hospitalization for progression of CHF |
| Rates and lengths of hospitalizations for management of adverse cardiovascular events |
| Rates and lengths of hospitalizations for any reason |
| Cardiac structure and function by echocardiographic examination including |
| LV end-diastolic and endsystolic diameters |
| LVEF |
| Left atrial dimensions |
| Degree of mitral and tricuspid regurgitation |
| Pulmonary artery systolic pressure |
| Intra- and interventricular mechanical delays |
| Rates of adverse events related to |
| LV lead |
| All leads |
| Success rate of the St Jude Medical LV lead implantation |
| Incidence of permanent AF, defined as the presence of AF in two consecutive ECGs |
| Primary |
| Survival after randomization |
| Functional capacity measured by the distance covered in the 6-MHW test at 24 months |
| QoL measured by the Minnesota Living With Heart Failure questionnaire at 24 months |
| Secondary |
| Functional capacity measured by the distance covered in the 6-MHW test at 12 months |
| QoL measured by the Minnesota Living With Heart Failure questionnaire at 12 months |
| Rate and duration of hospitalization for progression of CHF |
| Rates and lengths of hospitalizations for management of adverse cardiovascular events |
| Rates and lengths of hospitalizations for any reason |
| Cardiac structure and function by echocardiographic examination including |
| LV end-diastolic and endsystolic diameters |
| LVEF |
| Left atrial dimensions |
| Degree of mitral and tricuspid regurgitation |
| Pulmonary artery systolic pressure |
| Intra- and interventricular mechanical delays |
| Rates of adverse events related to |
| LV lead |
| All leads |
| Success rate of the St Jude Medical LV lead implantation |
| Incidence of permanent AF, defined as the presence of AF in two consecutive ECGs |
Methods
Study design
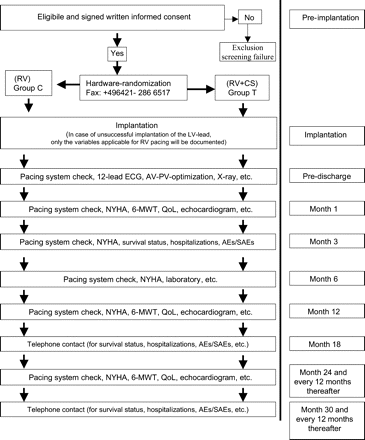
BioPace has been designed as a controlled, randomized, single-blind, parallel group trial. At least 80 international medical centres will enrol 1200 patients over an estimated recruitment period of 40 months. A biventricular pacing group will be compared with a group of recipients of conventional RV pacing systems. The study design is depicted in Figure 1.
Flow diagram of study enrollment, randomization and follow-up. CS, coronary sinus; MWT, minute walk test; AE, adverse event; SAE, serious adverse event.
Inclusion and exclusion criteria
The study inclusion and exclusion criteria are detailed in Tables 2 and 3. All patients have a class I indication for permanent ventricular pacing23 and a high likelihood of being paced at the ventricular level at least 66% of the time, regardless of the pre-implant spontaneous QRS-complex duration and morphology. Patients with implanted ICDs or patients who are considered for implantation of an ICD due to arrhythmia indication are excluded from the study. However, ICD implant for primary prevention of SCD in patients with LVEF≤35% will be allowed in accordance with the actual guidelines for the implantation of arrhythmia devices.23
Inclusion criteria
| Indications for ventricular pacing with high likelihood of predominantly paced ventricular beats: |
| Fixed third degree AV block |
| Type I (Mobitz I) second degree AV block combined with long (P–Q interval) |
| Type II (Mobitz II) second degree AV-block |
| First degree AV block and indication for ventricular pacing, for example, prolonged HV interval |
| Sick-sinus-syndrome with symptomatic sinus bradycardia or sinus arrest combined with long P–Q interval |
| Permanent AF, flutter, or atrial tachycardia, with a spontaneous resting ventricular rate ≤60/min |
| Permanent AF, flutter, or atrial tachycardia, with a spontaneous resting ventricular rate ≤75/min, and planned initiation or increase of drug therapy with heart rate slowing effect after pacemaker implantation |
| AF and planned AV junctional ablation |
| Indications for ventricular pacing with high likelihood of predominantly paced ventricular beats: |
| Fixed third degree AV block |
| Type I (Mobitz I) second degree AV block combined with long (P–Q interval) |
| Type II (Mobitz II) second degree AV-block |
| First degree AV block and indication for ventricular pacing, for example, prolonged HV interval |
| Sick-sinus-syndrome with symptomatic sinus bradycardia or sinus arrest combined with long P–Q interval |
| Permanent AF, flutter, or atrial tachycardia, with a spontaneous resting ventricular rate ≤60/min |
| Permanent AF, flutter, or atrial tachycardia, with a spontaneous resting ventricular rate ≤75/min, and planned initiation or increase of drug therapy with heart rate slowing effect after pacemaker implantation |
| AF and planned AV junctional ablation |
HV, His-ventricular.
Inclusion criteria
| Indications for ventricular pacing with high likelihood of predominantly paced ventricular beats: |
| Fixed third degree AV block |
| Type I (Mobitz I) second degree AV block combined with long (P–Q interval) |
| Type II (Mobitz II) second degree AV-block |
| First degree AV block and indication for ventricular pacing, for example, prolonged HV interval |
| Sick-sinus-syndrome with symptomatic sinus bradycardia or sinus arrest combined with long P–Q interval |
| Permanent AF, flutter, or atrial tachycardia, with a spontaneous resting ventricular rate ≤60/min |
| Permanent AF, flutter, or atrial tachycardia, with a spontaneous resting ventricular rate ≤75/min, and planned initiation or increase of drug therapy with heart rate slowing effect after pacemaker implantation |
| AF and planned AV junctional ablation |
| Indications for ventricular pacing with high likelihood of predominantly paced ventricular beats: |
| Fixed third degree AV block |
| Type I (Mobitz I) second degree AV block combined with long (P–Q interval) |
| Type II (Mobitz II) second degree AV-block |
| First degree AV block and indication for ventricular pacing, for example, prolonged HV interval |
| Sick-sinus-syndrome with symptomatic sinus bradycardia or sinus arrest combined with long P–Q interval |
| Permanent AF, flutter, or atrial tachycardia, with a spontaneous resting ventricular rate ≤60/min |
| Permanent AF, flutter, or atrial tachycardia, with a spontaneous resting ventricular rate ≤75/min, and planned initiation or increase of drug therapy with heart rate slowing effect after pacemaker implantation |
| AF and planned AV junctional ablation |
HV, His-ventricular.
Exclusion criteria
| Implanted ICD or planned implantation of an ICD due to arrhythmia indication. |
| Status 1 for cardiac transplantation (ICU bound, requiring pharmacological or mechanical support) and likelihood to be transplanted within 2 years |
| Implanted prosthetic tricuspid valve |
| Severe musculo-skeletal disorder |
| Age <18 years |
| Ongoing or planned pregnancy in the next 6 months |
| Current participation or participation within 30 days in another clinical study |
| Life expectancy <6 months |
| Inability to understand or complete the QoL questionnaire |
| Implanted ICD or planned implantation of an ICD due to arrhythmia indication. |
| Status 1 for cardiac transplantation (ICU bound, requiring pharmacological or mechanical support) and likelihood to be transplanted within 2 years |
| Implanted prosthetic tricuspid valve |
| Severe musculo-skeletal disorder |
| Age <18 years |
| Ongoing or planned pregnancy in the next 6 months |
| Current participation or participation within 30 days in another clinical study |
| Life expectancy <6 months |
| Inability to understand or complete the QoL questionnaire |
ICU, intensive care unit.
Exclusion criteria
| Implanted ICD or planned implantation of an ICD due to arrhythmia indication. |
| Status 1 for cardiac transplantation (ICU bound, requiring pharmacological or mechanical support) and likelihood to be transplanted within 2 years |
| Implanted prosthetic tricuspid valve |
| Severe musculo-skeletal disorder |
| Age <18 years |
| Ongoing or planned pregnancy in the next 6 months |
| Current participation or participation within 30 days in another clinical study |
| Life expectancy <6 months |
| Inability to understand or complete the QoL questionnaire |
| Implanted ICD or planned implantation of an ICD due to arrhythmia indication. |
| Status 1 for cardiac transplantation (ICU bound, requiring pharmacological or mechanical support) and likelihood to be transplanted within 2 years |
| Implanted prosthetic tricuspid valve |
| Severe musculo-skeletal disorder |
| Age <18 years |
| Ongoing or planned pregnancy in the next 6 months |
| Current participation or participation within 30 days in another clinical study |
| Life expectancy <6 months |
| Inability to understand or complete the QoL questionnaire |
ICU, intensive care unit.
Stratification and balanced randomization
Major prognostic factors that may influence the primary objectives are LVEF24, presence of AF,25,26 presence of complete LBBB,4,9 and NYHA functional class.20 In this study, the NYHA class is not recorded at the time of randomization, as patients may be confined to bed rest because of bradycardia. Furthermore, symptoms attributable to bradycardia vs. LV dysfunction may be difficult to separate.
Randomization results will be distributed by facsimile from the independent Coordinating Centre for Clinical Trials at Philipps-University Marburg, Germany. A balanced randomization is used according to the following stratifying scheme: Prior to randomization of an eligible patient, the information whether the patient is scheduled for ICD implant or not is documented. For balanced randomization and statistical analysis, one stratum was added by splitting up the stratum LVEF≤35% into LVEF≤35% with ICD implant and LVEF≤35% with implantation of a low-voltage (pacemaker) system. The previously existing stratum LVEF≤35% will be pooled with the stratum LVEF≤35% without ICD implant.
LVEF: (i) ≤35%, (ii) between 36 and 50%, (iii) >50%
AF: present or absent
ICD implant or implantation of a low-voltage (pacemaker) system.
Unpaced QRS duration: (i) ≤120 ms, (ii) between 121 and 150 ms, (iii) >150 ms
LBBB during spontaneous rhythm: present or absent
Enrolling centre
Pacing systems
Pulse generators
Patients assigned to biventricular pacing receive the Model 5510 Frontier 3×2™ (St Jude Medical Inc., Sylmar, CA, USA) triple-chamber pacemaker or newer models. Depending on whether sinus rhythm or chronic AF is present, patients randomized to the RV pacing group receive conventional single or dual-chamber pulse generators manufactured by St Jude Medical Inc.
Implantable cardioverter defibrillators
The Epic™ HF (Model V-339) or any other in terms of study relevant functions comparable with St Jude Medical CRT device with ICD backup will be used as the triple-chamber stimulation device in patients randomized to biventricular pacing who have been selected for the implantation of an ICD as an antibradycardia pacing device. No atrial lead will be implanted in patients with permanent AF in order to avoid procedural differences compared with the control group.
Depending on the presence or absence of sinus rhythm the Epic DR (Model V-233) or the Epic +VR (Model V-196) or any other in terms of study relevant functions comparable with St Jude Medical ICD will be used as stimulation device in patients randomized to RV pacing who have been selected for the implantation of an ICD. All ICDs will be programmed as ‘shock-only devices’ so that shocks will be delivered for ventricular fibrillation and fast, haemodynamically not tolerated, malignant ventricular tachycardias. The use of antitachycardia pacing therapies is not allowed under this protocol. This is crucial for an additional secondary data analysis in which every appropriate shock will be counted as an event of death.
AV synchronization
The individual AV delay is optimized in both study groups, for VDD and for DDD pacing, using preferably an electrocardiogram-based or echocardiogram-based27 method. The dual- and triple-chamber pulse generators as well as the corresponding ICD devices allow the programming of different AV intervals for atrial-triggered ventricular stimulation (PV delay) and for AV sequential pacing (AV delay).
Study-protocol for upgrade from RV to biventricular pacing
In the RV-paced group, a patient may be upgraded to a biventricular system if one or both of the following criteria are fulfilled: (i) the patient has been hospitalized three times for management of CHF progression to NYHA functional class IV since the beginning of the trial, (ii) the patient has become dependent on intravenous inotropic support and does not tolerate its withdrawal. With respect to the primary ‘survival’ endpoint, patients who need an upgrade are classified as ‘deceased’, as they were refractory to standard CHF therapy and had access to no other therapeutic option.
Lead system
The Model 1055 K Aescula™ or Model 1056 K QuickSite™ Left Heart Lead (St Jude Medical Inc.) are the LV leads used for this study. The model 1056T or newer St Jude Medical LV leads may be used when they become available. The use of bipolar leads is recommended. The RV lead is preferably placed at the RV apex, though other implantation sites are allowed. The LV lead is implanted transvenously via the coronary sinus, with a view to reach a lateral or postero-lateral LV segment.27 Should these sites be unattainable, the lead may be implanted elsewhere, as long as biventricular pacing is associated with a narrower QRS complex than RV pacing. An epicardial LV lead implantation is allowed if the transvenous approach is unsuccessful. Any commercially available ICD lead may be used, although the use of leads manufactured by St Jude Medical Inc. is encouraged.
Randomization
Screened patients who met the inclusion criteria and have given their informed consent are randomly assigned within 24 h to the implantation of a biventricular pacing or ICD system (treatment group) vs. implantation of a pacemaker or ICD for conventional RV pacing (control group) and undergo the implantation procedure within ≤5 workdays. The decision whether a pacemaker or an ICD device will be implanted is left to the discretion of the physician. Follow-up visits are scheduled at 1, 3 (optional depending on new devices), 6, and 12 months of follow-up and every 12 months thereafter.
Study organization
Echocardiographic recordings and core analyses
Echocardiographic examinations are performed at each study site prior to pacemaker implantation at 1, 12, and 24 months after implantation and at the end of the study. All recordings are stored on super-VHS videotapes or on DVD/CD-ROM. After the on-site analysis of the pre-implant echocardiogram, the data are forwarded to the echocardiographic core laboratory for central analysis (appendix).
Independent Safety Review Committee
The Independent Safety Review Committee (ISRC) is composed of three experts who are not investigators in this study (appendix). The ISRC may request interim analyses if an excessive rate of adverse events is suspected in one or the other study group. This committee is responsible for classifying in a blinded fashion all events that may constitute an endpoint or adverse event. The ISRC may recommend the premature termination of the trial to the sponsor. So far, the ISRC has not made any specific comments.
Statistical considerations
Confirmatory statistical analysis
The analysis of the effectiveness of biventricular stimulation will be based on (i) patient survival, (ii) distance covered during the 6-MHW at 24 months, and (iii) QoL at 24 months, ascertained by the Minnesota© Living with Heart Failure Questionnaire score. The log-rank and Wilcoxon–Mann–Whitney test will be used with a view to establish the superiority of biventricular stimulation over conventional RV pacing. Two-sided stratified tests with eight strata will be carried out, considering the main prognostic factors LVEF in combination with ICD implant (≤35% with ICD implant, ≤35% without ICD implant, >35% and ≤50%, and >50%) and AF (PRESENT/ABSENT). The intention-to-treat principle will be applied for the primary analysis.
Strongly controlling for multiplicity at an overall, two-sided significance level of α=5%, the closed testing principle by Marcus and Peritz will be used.28 The global intersection hypothesis will be tested by the three elementary hypotheses at a level of (i) 2.5% (survival), (ii) 1.25% (6-MHW at 24 months), and (iii) 1.25% (Minnesota at 24 months). In a second stage, the three intersection hypotheses will be tested by the elementary hypotheses at a level of 2.5% each. Finally, the elementary hypotheses will be tested in a third stage at a level of 5% each. In the case of a significant improvement of functional capacity or QoL, the superiority of biventricular stimulation will only be claimed if there is no statistically significant or trend towards decrease in survival.
The study was initially planned as a fixed sample design. However, the data will be monitored statistically. Interim analyses and design adaptations based on grouped data may be performed at any time during the course of the trial, according to the conditional rejection probability principle.29–32
Sample size and power considerations
Sample size was calculated on the basis of expected survival time, a two-sided significance level of 2.5%, the log-rank test assuming proportional hazards, and the method of Schoenfeld and Richter32 assuming nearly exponential survival curves and a uniform recruitment of patients. We expect a median survival time of 5 years for the RV-paced group and, furthermore, we considered an increase in survival time from 5 to 7 years by biventricular pacing to be clinically relevant. With a recruitment period of 2 years and a follow-up period of 3 years, at least 382 events are required to achieve a power of 80%. The numbers have been adjusted for 5% of randomized patients not fulfilling the criterion of ≥66% ventricular pacing at 1 month after implantation and for an assumed 15% missing survival information due to loss of follow-up. Consequently, we have planned for an overall recruitment of 1200 patients, 600 assigned to each group. The assumption of a median survival time of 5 years in this study corresponds to a hazard ratio of 5/7=0.714, which is not unrealistic if the data of, for example, the Care-HF study (HR=0.64)33 are taken into account. In contrast, this appears as a rather conservative estimation, even if the effect of biventricular pacing in patients with LBBB was considered to be greater than the effect of biventricular pacing compared with RV pacing.34,35 Real median survival time of the recruited patients may turn out to be either shorter or longer than expected. This will determine the follow-up period that is needed to achieve the required number of deaths to be observed. In this context, it is noteworthy that not only the sample size but also the number of events to be observed can be adapted. This is the advantage of the possibility to perform interim analyses with design adaptations based on grouped data at any time during the course of the trial.30,31
Diary
The study started in year 2003 and it was initially expected to complete patient enrolment by the end of 2005. Patient recruitment as well as to some extent also centre recruitment, however, appeared to be slower than expected. The main reasons were as follows. At present, more than 700 patients have been included in the study. Recently, patient enrolment has increased considerably and patient inclusion is expected to be completed by the end of 2006.
The implantation of a biventricular device as an ad hoc procedure in patients with AV block turned out not to be per se comparable with scheduled CRT system implants in stable patients with chronic heart failure and LBBB. Urgent biventricular implants can sometime be somewhat challenging, especially in terms of hospital logistics.
Because BioPace patients have a routine antibradycardia pacing indication and in order to let the study take place under ‘real-world’ conditions, also centres with up to now lacking biventricular experience have been included: especially during the initial phase of the study, some of these centres had a lower recruitment rate than expected.
The recruitment of centres was probably delayed because some of the potential investigators while ignoring potential negative sequelae from biventricular implantations under urgent conditions might have tended to anticipate positive prognostic effects of biventricular compared with RV pacing from positive mechanistic effects that have been found in smaller studies with symptomatic or mechanistic endpoints such as the PAVE study.19
During the period when the BioPace study was launched, the scientific context of the study with respect to the important role of ICDs in primary prevention of SCD21,22 was not only heterogeneous but also changing in the participating countries, when the initial study protocol did not offer the possibility to implant ICDs as pacing devices. This turned out to be slowing down not only the recruitment of new centres but also the inclusion of patients in already collaborating centres, because some investigators already accepted the results of the MADIT II study21 and the SCD-Heft studies22 to implant ICDs instead of pacemakers in patients with need for chronic ventricular stimulation and severely impaired LV function. Therefore, in August 2005, the ICD amendment to the original study protocol was made to allow them to be implanted as pacing devices in patients who fulfil the inclusion criteria of the BioPace study and who present with severe LV dysfunction.
Appendix
Study Steering Committee: Jean-Jacques Blanc, MD (Chair), Luc De Roy, MD, Reinhard C. Funck, MD (Principal Investigator), Maurizio Lunati, MD, Vince Paul, MD, Christophe Bailleul, PhD (Sponsor Representative)
Independent Safety Review Committee: Christophe Leclercq, MD, Cecilia Linde, MD (Chair), Hans J. Trampisch, PhD
Echocardiographic Core Laboratory: Alison Duncan, MD, Michael Y. Henein (Director), MD, Wei Li, MD