Abstract
Many children and adolescents with cancer receive chemotherapeutic agents that are cardiotoxic. Thus, while survival rates in this population have improved for some cancers, many survivors may experience acute or chronic cardiovascular complications that can impair their quality of life years after treatment. In addition, cardiac complications of treatment lead to reductions in dose and duration of chemotherapy regimens, potentially compromising clinical efficacy.
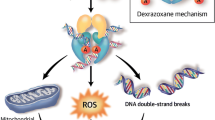
Anthracyclines are well known for their cardiotoxicity, and alkylating agents, such as cyclophosphamide, ifosfamide, cisplatin, busulfan, and mitomycin, have also been associated with cardiotoxicity. Other agents with cardiac effects include vinca alkaloids, fluorouracil, cytarabine, amsacrine, and asparaginase and the newer agents, paclitaxel, trastuzumab, etoposide, and teniposide. The heart is relatively vulnerable to oxidative injuries from oxygen radicals generated by chemotherapy.
The cardiac effects of these drugs include asymptomatic electrocardiographic abnormalities, blood pressure changes, arrhythmias, myocarditis, pericarditis, cardiac tamponade, acute myocardial infarction, cardiac failure, shock, and long-term cardiomyopathy. These effects may occur during or immediately after treatment or may not be apparent until months or years after treatment.
Mild myocardiocyte injury from chemotherapy may be of more concern in children than in adults because of the need for subsequent cardiac growth to match somatic growth and because survival is longer in children. Primary prevention is therefore important. Patients should be educated about the cardiotoxic risks of treatment and the need for long-term cardiac monitoring before chemotherapy is begun. Cardiotoxicity may be prevented by screening for risk factors, monitoring for signs and symptoms during chemotherapy, and continuing follow-up that may include electrocardiographic and echocardiographic studies, angiography, and measurements of biochemical markers of myocardial injury.
Secondary prevention should aim to minimize progression of left ventricular dysfunction to overt heart failure. Approaches include altering the dose, schedule, or approach to drug delivery; using analogs or new formulations with fewer or milder cardiotoxic effects; using cardioprotectants and agents that reduce oxidative stress during chemotherapy; correcting for metabolic derangements caused by chemotherapy that can potentiate the cardiotoxic effects of the drug; and cardiac monitoring during and after cancer therapy. Avoiding additional cardiotoxic regimens is also important in managing these patients. Treating the adverse cardiac effects of chemotherapy will usually be dependent on symptoms or will depend on the anticipated cardiovascular effects of each regimen. Treatments include diuresis, afterload reduction, β-adrenoceptor antagonists, and improving myocardial contractility.


Similar content being viewed by others
Notes
The use of trade names is for product identification purposes only and does not imply endorsement.
References
Simbre VC, Adams MJ, Deshpande SS, et al. Cardiomyopathy caused by antineoplastic therapies. Curr Treat Options Cardiovasc Med 2001; 3: 493–505
Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol 2004; 22(15): 3139–48
Adams MJ, Lipshultz SE, Schwartz C, et al. Radiation-associated cardiovascular disease: manifestations and management. Semin Radiat Oncol 2003; 13(3): 346–56
Adams MJ, Hardenbergh PH, Constine LS, et al. Radiation-associated cardiovascular disease. Crit Rev Oncol Hematol 2003; 45(1): 55–75
Cuthbertson DD, Epstein ST, Lipshultz SE, et al. Anthracycline cardiotoxicity in children with cancer [abstract]. Circulation 1994; 90(4): 50
Office of Cancer Survivorship. National Cancer Institute workshop on long-term follow-up care programs for survivors of pediatric cancer, Niagara-on-the-Lake. 2002 Jun 25, Ontario
Gerwitz DA. Critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 1999; 57: 727–41
Goormaghtigh E, Huart P, Praet M, et al. Structure of the adriamycin-cardiolipin complex: role in mitochondrial toxicity. Biophys Chem 1990; 35(2-3): 247–57
Stone RM, Bridges KR, Libby P. Hematological-oncological disorders and cardiovascular disease. In: Braunwald E, Zipes DP, Libby P, et al., editors. Heart disease. 6th ed. Philadelphia (PA): WB Saunders Co, 2001: 2223–43
Yeh ET, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation 2004; 109(25): 3122–31
Horenstein MS, Vander Heide RS, L’Ecuyer TJ. Molecular basis of anthracycline-induced cardiotoxicity and its prevention. Mol Genet Metab 2000; 71(1-2): 436–44
Jeyaseelan R, Poizat C, Wu H. Molecular mechanisms of doxorubicin-induced cardiomyopathy: selective suppression of Reiske iron-sulfur protein, ADP/ATP translocase, and phosphofructokinase genes is associated with ATP depletion in rat cardiomyocytes. J Biol Chem 1997; 272(9): 5828–32
Arai M, Yoguchi A, Takizawa T. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca-ATPase gene transcription. Mol Med 2000; 86(8): 8–14
Giantris A, Abdurrahman L, Hinkle A, et al. Anthracycline-induced cardiotoxicity in children and young adults. Crit Rev Oncol Hematol 1998; 27(1): 53–68
Mott MG. Anthracycline cardiotoxicity and its prevention: challenges and opportunities. Pediatr Oncol 1997; 824: 221–8
Buzdar AU, Marcus C, Smith TL, et al. Early and delayed clinical cardiotoxicity of doxorubicin. Cancer 1985; 55(12): 2761–5
Krischer JP, Epstein S, Cuthbertson DD, et al. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the Pediatric Oncology Group experience. J Clin Oncol 1997; 15(4): 1544–52
Praga C, Bretta G, Vigo PL, et al. Adriamycin cardiotoxicity: a survey of 1273 patients. Cancer Treat Rep 1979; 63: 827–34
Jain KK, Casper ES, Geller NL, et al. A prospective randomized comparison of epirubicin and doxorubicin in patients with advanced breast-cancer. J Clin Oncol 1985; 3(6): 818–26
Grenier MA, Lipshultz SE. Epidemiology of anthracycline cardiotoxicity in children and adults. Semin Oncol 1998; 25(4): 72–85
Lipshultz SE, Colan SD, Gelber RD, et al. Late cardiac effects of doxorubicin therapy for acute lymphoblastic-leukemia in childhood. N Engl J Med 1991; 324(12): 808–15
Lipshultz SE, Lipsitz SR, Mone SM, et al. Female sex and higher drug dose as risk-factors for late cardiotoxic effects of doxorubicin therapy for childhood-cancer. N Engl J Med 1995; 332(26): 1738–43
Lipshultz SE, Lipsitz SR, Sallan SE, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol 2005; 23(12): 2629–36
Adams MJ, Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer 2005; 44(7): 600–6
Dresdale A, Bonow RO, Wesley R, et al. Prospective evaluation of doxorubicin-induced cardiomyopathy resulting from postsurgical adjuvant treatment of patients with soft-tissue sarcomas. Cancer 1983; 52(1): 51–60
Gottdiener JS, Appelbaum FR, Ferrans VJ, et al. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med 1981; 141(6): 758–63
Chakko S, Woska D, Martinez H, et al. Clinical, radiographic and hemodynamic correlations in chronic congestive heart failure: conflicting result may lead to inappropriate care. Am J Med 1991; 90: 353–6
Lipshultz SE, Sanders SP, Goorin AM, et al. Monitoring for anthracycline cardiotoxicity. Pediatrics 1994; 93(3): 433–7
Ottlinger ME, Pearsall L, Rifai N, et al. New developments in the biochemical assessment of myocardial injury in children: troponins T and I as highly sensitive and specific markers of myocardial injury. Prog Pediatr Cardiol 1997; 8(2): 71–81
Lipshultz SE, Rifai N, Sallan SE, et al. Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 1997; 96(8): 2641–8
Lipshultz SE, Sallan SE, Dalton V, et al. Elevated serum cardiac troponin T as a marker for active cardiac injury during therapy for childhood acute lymphoblastic leukemia [abstract]. J Am Coll Cardiol 2001; 37: 466A
Billingham ME, Mason JW, Bristow MR, et al. Anthracycline cardiomyopathy monitored by morphological changes. Cancer Treat Rep 1978; 62: 865–72
Isner JM, Ferrans VJ, Cohen SR, et al. Clinical and morphologic cardiac findings after anthracycline chemotherapy. Am J Cardiol 1983; 51: 1167–74
Druck MN, Gulenchyn KY, Evans WK, et al. Radionuclide angiography and endomyocardial biopsy in the assessment of doxorubicin cardiotoxicity. Cancer 1998; 53: 1667–74
Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med 1979; 91: 710–7
Legha SS, Benjamin RS, Mackay B, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous-infusion. Ann Intern Med 1982; 96(2): 133–9
Torti FM, Bristow MR, Howes AE, et al. Reduced cardiotoxicity of doxorubicin delivered on a weekly schedule: assessment by endomyocardial biopsy. Ann Intern Med 1983; 99(6): 745–9
Weiss AJ, Metter GE, Fletcher WS, et al. Studies on adriamycin using a weekly regimen demonstrating its clinical effectiveness and lack of cardiac toxicity. Cancer Treat Rep 1976; 60(7): 813–22
Weiss AJ, Manthel RW. Experience with the use of adriamycin in combination with other anticancer agents using a weekly schedule with particular reference to lack of cardiac toxicity. Cancer 1977; 40: 2046–52
Chelobowski RT, Paroly WS, Pugh RP, et al. Adriamycin given as a weekly schedule without a loading course: clinically effective with reduced incidence of cardiotoxicity. Cancer Treat Rep 1980; 6: 447–51
Lipshultz SE, Giantris AL, Lipsitz SR, et al. Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 Acute Lymphoblastic Leukemia protocol. J Clin Oncol 2002; 20(6): 1677–82
Pihkala J, Saarinen UM, Lundstrom U, et al. Myocardial function in children and adolescents after therapy with anthracyclines and chest irradiation. Eur J Cancer 1996; 32A(1): 97–103
Torti FM, Bristow MM, Lum BL, et al. Cardiotoxicity of epirubicin and doxorubicin: assessment by endomyocardial biopsy. Cancer Res 1986; 46(7): 3722–7
Mengozzi G, Palagi C, Petronio AS, et al. The evaluation of cardiotoxicity of 4 epidoxorubicin at high doses. Cardiologia 1991; 36: 137–42
Lahtinen R, Kuikka J, Nousiainen T, et al. Cardiotoxicity of epirubicin and doxorubicin: a double-blind randomized study. Eur J Haematol 1991; 46(5): 301–5
Ganzina F. 4-epi-doxorubicin, a new analogue of doxorubicin: a preliminary overview of preclinical and clinical data. Cancer Treat Rev 1983; 10: 1–22
Cersosimo RJ, Hong WK. Epirubicin: a review of the pharmacology, clinical activity and adverse effects of an adriamycin analogue. J Clin Oncol 1986; 4: 425–39
Brambilla C, Rossi A, Bonfante V, et al. Phase-II study of doxorubicin versus epirubicin in advanced breast-cancer. Cancer Treat Rep 1986; 70(2): 261–6
Intini C, Sacchetti G. FEC vs FAC in advanced breast cancer: an Italian multicenter trial. In: Ishigami J, editor. Recent advances in chemotherapy. Tokyo: University of Tokyo Press, 1986: 1198–9
Cottin Y, Touzery C, Dalloz F, et al. Comparison of epirubicin and doxorubicin cardiotoxicity induced by low doses: evolution of the diastolic and systolic parameters studied by radionuclide angiography. Clin Cardiol 1998; 21: 665–70
Anderlini P, Benjamin RS, Wong FC, et al. Idarubicin cardiotoxicity: a retrospective study in acute myeloid-leukemia and myelodysplasia. J Clin Oncol 1995; 13(11): 2827–34
Creutzig U, Korholz D, Niemeyer CM, et al. Toxicity and effectiveness of high-dose idarubicin during AML induction therapy: results of a pilot study in children. Klin Padiatr 2000; 212(4): 163–8
Hasinoff BB, Reinders FX, Clark V. The enzymatic hydrolysis-activation of the adriamycin cardioprotective agent (+)-1,2-bias (3,5-dioxopiperazinyl-1-yl) propane. Drug Metab Dispos 1991; 19: 74–80
Iarussi D, Indolfi P, Casale F, et al. Recent advances in the prevention of anthracycline cardiotoxicity in childhood. Curr Med Chem 2001; 8: 1649–60
Hasinoff BB. Chemistry of dexrazoxane and analogues. Semin Oncol 1998; 25(4): 3–9
Seifert CF, Nesser ME, Thompson DF. Dexrazoxane in the prevention of doxorubicin-induced cardiotoxicity. Ann Pharmacother 1994; 28(9): 1063–72
Hasinoff BB, Kala SV. The removal of metal ions from transferrin, ferritin and ceruloplasmin by the cardioprotective agent ICRF-187 [(+)-1,2-bis (3,5-dioxopiperazinyl-1-yl) propane] and its hydrolysis product ADR-925. Agents Actions 1993; 39(1-2): 72–81
Ryan TP, Samokyszyn VM, Dellis S, et al. Effects of (+)-1,2-bis (3,5-dioxopiperazinyl-1-yl) propane (ADR-529) on iron-catalyzed lipid peroxidation. Chem Res Toxicol 1990; 3(4): 384–90
Herman EH, Ferrans VJ, Young RS, et al. Pretreatment with ICRF-187 allows a marked increase in the total cumulative dose of doxorubicin tolerated by beagle dogs. Drugs Exp Clin Res 1988; 14(90): 563–70
Herman EH, Ferrans VJ, Jordan W, et al. Reduction of chronic daunorubicin cardiotoxicity by ICRF-187 in rabbits. Res Commun Chem Pathol Pharmacol 1981; 31: 85–97
Herman EH, Ferrans VJ, Young RS, et al. Effect of pretreatment with ICRF-187 on the total cumulative dose of doxorubicin tolerated by beagle dogs. Cancer Res 1988; 48(23): 6918–25
Styczynski J, Wysocki M, Balwierz W, et al. Dexrazoxane has no impact on sensitivity of childhood leukemic blasts to daunorubicin. Leukemia 2002; 16(5): 820–5
Swain SM, Whaley FS, Gerber MC, et al. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin-containing therapy. J Clin Oncol 1997; 15(4): 1333–40
Lopez M, Vici P, Di Lauro G, et al. Randomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol 1998; 16: 86–92
Schuchter LM, Hensley ML, Meropol NJ, et al. 2002 update of recommendations for the use of chemotherapy and radiotherapy protectants: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol 2002; 20(12): 2895–903
Bu Lock FA, Gabriel HM, Oakhill A, et al. Cardioprotection by ICRF-187 against high dose anthracycline toxicity in children with malignant disease. Br Heart J 1993; 70(2): 185–8
Wexler LH, Andrich MP, Venzon D, et al. Randomized trial of the cardioprotective agent ICRF-187 in pediatric sarcoma patients treated with doxorubicin. J Clin Oncol 1996; 14(2): 362–72
Schiavetti A, Castello MA, Versacci P, et al. Use of ICRF-187 for prevention of anthracycline cardiotoxicity in children: preliminary results. Pediatr Hematol Oncol 1997; 14(3): 213–22
Lipshultz SE. Dexrazoxane for protection against cardiotoxic effects of anthracyclines in children. J Clin Oncol 1996; 14(2): 328–31
Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol 1997; 15(4): 1318–32
Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med 2004; 351(2): 145–53
Koenig J, Fischer M, Bulants E. Antioxidant status in patients on chronic hemodiolysis therapy: impact of parenteral selenium supplementation. Wien Klin Wochenschr 1997; 109(1): 13–9
Sies H. Oxidative stress: from basic research to clinical application. Am J Med 1991; 91: S31–8
Bast A, Haenen GRMM, Doelman CJA. Oxidants and antioxidants: state-of-the-art. Am J Med 1991; 91: S2–13
Ondreicka R, Beno I, Brancicova E. Relation between levels of vitamins C, E, A and beta carotene and activity of antioxidant enzymes in the blood. Bratisl Lek Listy 1998; 99(5): 250–4
Yen HC, Oberley TD, Vichitbandha S, et al. The protective role of manganese Superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest 1996; 98(5): 1253–60
Kang Y, Chen Y, Epstein P. Suppression of doxorubicin cardiotoxicity by overex-pression of catalase in the heart of transgenic mice. J Biol Chem 1996; 271(21): 12610–6
Kang YJ, Chen Y, Yu AD, et al. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest 1997; 100(6): 1501–6
He N, Singhal S, Srivastava S. Transfection of 4-hydroxynonenal metabolizing glutathione: S-transferase isozyme, mouse GSTA4-4, confers doxorubicin resistance to Chinese hamster ovary cells. Arch Biochem Biophys 1996; 333(1): 214–20
Halliwell B. Reactive oxygen species in living systems: source, biochemistry, and role in human disease. Am J Med 1991; 91: S14–22
Ferrari R, Ceconi C, Curello S, et al. Oxygen free-radicals and myocardial damage: protective role of thiol-containing agents. Am J Med 1991; 91: S95–105
Dorr R, Lagel K, McLean S. Cardioprotection of rat heart myocytes with amifostine (Ethyol®) and its free thiol, WR-1065, in vitro. Eur J Cancer 1996; 32A(4): 21–5
Shioji K, Kishimoto C, Nakamura H, et al. Overexpression of thioredoxin-1 in transgenic mice attenuates adriamycin-induced cardiotoxicity. Circulation 2002; 106(11): 1403–9
Siveski-Iliskovic N, Kaul N, Singal P. Probucol promotes endogenous antioxidants and provides protection against adriamycin-induced cardiomyopathy in rats. Circulation 1994; 89: 2829–35
Vora J, Khaw BA, Narula J, et al. Protective effect of butylated hydroxyanisole on adriamycin-induced cardiotoxicity. J Pharm Pharmacol 1996; 48(9): 940–4
Lipshultz SE, Lipsitz SR, Sallan SE, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol 2002; 20(23): 4517–22
The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med 1992; 327: 685–91
Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left-ventricular dysfunction after myocardial-infarction: results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992; 327(10): 669–77
Lipshultz SE, Colan SD. Cardiovascular trials in long-term survivors of childhood cancer. J Clin Oncol 2004; 22(5): 769–73
Fu LX, Bergh CH, Hoebeke J, et al. Effect of metoprolol on activity of beta-adrenoceptor coupled to guanine nucleotide binding regulatory proteins in adriamycin-induced cardiotoxicity. Basic Res Cardiol 1991; 86(2): 117–26
Mak IT, Weglicki WB. Protection by beta-blocking-agents against free radical-mediated sarcolemmal lipid-peroxidation. Circ Res 1988; 63(1): 262–6
Kramer JK, Mak IT, Freedman AM, et al. Propranolol reduces anoxia/reoxygenation-mediated injury of adult myocytes through an anti-radical mechanism. J Mol Cell Cardiol 1991; 23: 1231–44
Liu XK, Engelman RM, Agrawal HR, et al. Preservation of membrane phospholipids by propranolol, pindolol, and metoprolol: a novel mechanism of action of beta-blockers. J Mol Cell Cardiol 1991; 23(10): 1091–100
Noori A, Lindenfeld J, Wolfel E, et al. Beta-blockade in adriamycin-induced cardiomyopathy. J Card Fail 2000; 6(2): 115–9
Lipshultz SE, Vlach SA, Lipsitz SR, et al. Cardiac changes associated with growth hormone therapy among children treated with anthracyclines. Pediatrics 2005; 115: 1613–22
Miller TL, Horgan S, Lipshultz SE. Exercise rehabilitation of pediatric patients with cardiovascular disease. Prog Pediatr Cardiol 2005; 20: 27–37
Goldberg MA, Antin JH, Guinan EC, et al. Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor. Blood 1986; 68(5): 1114–8
Steinherz LJ, Steinherz PG, Mangiacasale D, et al. Cardiac changes with cyclophosphamide. Med Pediatr Oncol 1981; 9(5): 417–22
Buja LM, Ferrans VJ, Graw RG. Cardiac pathologic findings in patients treated with bone-marrow transplantation. Hum Pathol 1976; 7(1): 17–45
Kupari M, Volin L, Suokas A, et al. Cardiac involvement in bone-marrow transplantation: electrocardiographic changes, arrhythmias, heart-failure and autopsy findings. Bone Marrow Transplant 1990; 5(2): 91–8
Slavin RE, Millan JC, Mullins GM. Pathology of high dose intermittent cyclophosphamide therapy. Hum Pathol 1975; 6(6): 693–709
O’Connell TX, Berenbaum MC. Cardiac and pulmonary effects of high doses of cyclophosphamide and isophosphamide. Cancer Res 1974; 34: 1586–91
Applebaum FR, Strauchen JA, Graw GR, et al. Acute lethal carditis caused by high dose combination chemotherapy. Lancet 1976; I: 58–62
Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf 2000; 22(4): 263–302
Trigg ME, Finlay JL, Bozdech M, et al. Fatal cardiac toxicity in bone-marrow transplant patients receiving cytosine-arabinoside, cyclophosphamide, and total-body irradiation. Cancer 1987; 59(1): 38–42
Friedman HS, Colvin OM, Aisaka K, et al. Glutathione protects cardiac and skeletal muscle from cyclophosphamide-induced toxicity. Cancer Res 1990; 50(8): 2455–62
Braverman AC, Antin JH, Plappert MT, et al. Cyclophosphamide cardiotoxicity in bone-marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol 1991; 9(7): 1215–23
La Bianca R, Beretta G, Clerici M, et al. Cardiac toxicity of 5-fluorouracil: a study of 1083 patients. Tumori 1982; 68: 505–10
Gradishar WJ, Vokes EE. 5-fluorouracil cardiotoxicity: a critical review. Ann Oncol 1990; 1(6): 409–14
Jakubowski AA, Kemeny N. Hypotension as a manifestation of cardiotoxicity in three patients receiving cisplatin and 5-fluorouracil. Cancer 1988; 62: 266–9
Rezkalla S, Kloner RA, Ensley J, et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: a prospective study. J Clin Oncol 1989; 7(4): 509–14
Robben NC, Pippas AW, Moore JO. The syndrome of 5-fluorouracil cardiotoxicity: an elusive cardiopathy. Cancer 1993; 71(2): 493–509
Pottage A, Holt S, Ludgate S, et al. Fluorouracil cardiotoxicity [letter]. BMJ 1978; 1(6112): 547
Fajardo LF, Stewart JR. Pathogenesis of radiation-induced myocardial fibrosis. Lab Invest 1973; 29(2): 244–57
Lindsay E, Enterman C, Ellis EE, et al. Aortic atherosclerosis in the dog after localized aortic irradiation with electrons. Circ Res 1962; 10: 61–7
Patel B, Kloner RA, Ensley J, et al. 5-fluorouracil cardiotoxicity: left-ventricular dysfunction and effect of coronary vasodilators. Am J Med Sci 1987; 294(4): 238–43
May D, Wandl U, Beher R, et al. Cardiac side effects of 5-fluorouracil. Dtsch Med Wochenschr 1990; 115: 618–21
Eskilsson J, Albertsson M. Failure of preventing 5-fluorouracil cardiotoxicity by prophylactic treatment with verapamil. Acta Oncol 1990; 29(8): 1001–3
Schober C, Papageorgiou E, Harstrick A, et al. Cardiotoxicity of 5-fluorouracil in combination with folinic acid in patients with gastrointestinal cancer. Cancer 1993; 72(7): 2242–7
Oleksowicz L, Bruckner HW. Prophylaxis of 5-fluorouracil-induced coronary vasospasm with calcium-channel blockers. Am J Med 1988; 85(5): 750–1
Gormley PE, Sethi VS, Cysyk RL. Interaction of 4′(9-acridinylamino) methanesulfon-m-anisidide with DNA and inhibition of oncornavirus reverse-transcriptase and cellular nucleic-acid polymerases. Cancer Res 1978; 38(5): 1300–6
Weiss RB, Grillolopez AJ, Marsoni S, et al. Amsacrine-associated cardiotoxicity: an analysis of 82 cases. J Clin Oncol 1986; 4(6): 918–28
Weiss RB, Moquin D, Adams JD, et al. Electrocardiographic abnormalities induced by amsacrine. Cancer Chemother Pharmacol 1983; 10: 133–4
Willemze R, Zwaan FE, Colpin G, et al. High-dose cytosine-arabinoside in the management of refractory acute-leukemia. Scand J Haematol 1982; 29(2): 141–6
Conrad ME. Cytarabine and cardiac-failure. Am J Hematol 1992; 41(2): 143–4
Andersson BS, Cogan BM, Keating MJ, et al. Subacute pulmonary failure complicating therapy with high-dose Ara-C in acute-leukemia. Cancer 1985; 56(9): 2181–4
Haupt HM, Hutchins GM, Moore GW. Ara-C lung: noncardiogenic pulmonary-edema complicating cytosine-arabinoside therapy of leukemia. Am J Med 1981; 70(2): 256–61
Donehower RC, Karp JE, Burke PJ. Pharmacology and toxicity of high-dose cytarabine by 72-hour continuous infusion. Cancer Treat Rep 1986; 70(9): 1059–65
Chiche D, Pico JL, Bernaudin JF, et al. Pulmonary-edema and shock after high-dose aracytine-C for lymphoma: possible role of TNF-alpha and PAF. Eur Cytokine Netw 1993; 4(2): 147–51
Castleberry RP, Crist WM, Holbrook T, et al. The cytosine arabinoside syndrome. Med Pediatr Oncol 1981; 9: 257–64
Vaickus L, Letendre L. Pericarditis induced by high-dose cytarabine therapy. Arch Intern Med 1984; 144(9): 1868–9
Williams SF, Larson RA. Hypersensitivity reaction to high-dose cytarabine. Br J Haematol 1989; 73(2): 274–5
Rowinsky EK, Cazenave LA, Donehower RC. Taxol®: a novel investigational antimicrotubule agent. J Natl Cancer Inst 1990; 82(15): 1247–59
Rowinsky EK, Donehower RC. Drug therapy: paclitaxel (Taxol®). N Engl J Med 1996; 332: 1004–14
Jordan MA, Toso RJ, Thrower D, et al. Mechanism of mitotic block and inhibition of cell-proliferation by Taxol® at low concentrations. Proc Natl Acad Sci U S A 1993; 90(20): 9552–6
Tishler RB, Schiff PB, Geard CR, et al. Taxol®: a novel radiation sensitizer. Int J Radiat Oncol Biol Phys 1992; 22(3): 613–7
Tishler RB, Geard CR, Hall EJ, et al. Taxol® sensitizes human astrocytoma-cells to radiation. Cancer Res 1992; 52(12): 3495–7
Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from Taxol®. J Clin Oncol 1990; 8(7): 1263–8
Rowinsky EK, Mcguire WP, Guarnieri T, et al. Cardiac disturbances during the administration of Taxol®. J Clin Oncol 1991; 9(9): 1704–12
Arbuck SG, Strauss H, Rowinsky E, et al. A reassessment of the cardiac toxicity associated with Taxol®. Monogr Natl Cancer Inst 1993; 15: 117–30
Mcguire WP, Rowinsky EK, Rosenshein NB, et al. Taxol®: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med 1989; 111(4): 273–9
Rowinsky EK, Gilbert MR, Mcguire WP, et al. Sequences of Taxol® and cisplatin: a phase-I and pharmacological study. J Clin Oncol 1991; 9(9): 1692–703
Gianni L, Straneo G, Capri F, et al. Optimal dose and sequence finding study of paclitaxel (P) by 3 hour infusion with bolus doxorubicin (D) in untreated metastatic breast cancer patients [letter]. Proc Am Soc Clin Oncol 1994; 13: 74
O’Dwyer PJ, Johnson SW, Hamilton TC. Cisplatin and its analogues. In: Devita Jr VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. Philadelphia (PA): Lippincott-Raven, 1997: 418–32
Talley RW, Obryan RM, Gutterma JU, et al. Clinical evaluation of toxic effects of cis-diamminedichloroplatinum (Nsc-119875): phase-1 clinical-study. Cancer Chemother Rep 1973; 57(4): 465–71
Wiltshaw E, Carr B. Cis-platinum (II) diamminedichloride: clinical experience of the Royal Marsden Hospital and Institute of Cancer Research. In: Connors TA, Roberts JJ, editors. Platinum coordination complexes in cancer chemotherapy. London: Springer, 1974: 178–82
Hashimi LA, Khalyl MF, Salem PA. Supraventricular tachycardia: a probable complication of platinum treatment. Oncology 1984; 41(3): 174–5
Shaeppi U, Heyman IA, Fleschman RW. Cis-diamminedichloroplatinum (II) preclinical evaluation of intravenous injection in dogs, monkeys and mice. Toxicol Appl Pharmacol 1973; 25: 230–41
Doll DC, List AF, Greco FA, et al. Acute vascular ischemic events after cisplatin-based combination chemotherapy for germ-cell tumors of the testis. Ann Intern Med 1986; 105(1): 48–51
Talcott JA, Herman TS. Acute ischemic vascular events and cisplatin. Ann Intern Med 1987; 107(1): 121–2
Licciardello JTW, Moake JL, Rudy CK, et al. Elevated plasma Von Willebrand factor levels and arterial occlusive complications associated with cisplatin-based chemotherapy. Oncology 1985; 42(5): 296–300
Bodensteiner DC. Fatal coronary-artery fibrosis after treatment with bleomycin, vinblastine, and cis-platinum. South Med J 1981; 74(7): 898–9
Jackson AM, Rose BD, Graff LG, et al. Thrombotic microangiopathy and renal failure associated with antineoplastic chemotherapy. Ann Intern Med 1987; 101: 121–2
Vogelzang NJ, Torkelson JL, Kennedy BJ. Hypomagnesemia, renal dysfunction, and Raynaud phenomenon in patients treated with cisplatin, vinblastine, and bleomycin. Cancer 1985; 56(12): 2765–70
Hinkle AS, Proukou C, French CA, et al. A clinic-based, comprehensive care model for studying late effects in long-term survivors of pediatric illnesses. Pediatrics 2004; 113(4 Suppl.): 1141–5
Acknowledgments
This review was supported in part by the National Institutes of Health, Bethesda, MD (CA-79060, HL59837, HR96041, CA68484, HL53392, HL78522, HL72705), Lance Armstrong Foundation, and the Children’s Cardiomyopathy Foundation. Dr Lipshultz has been a consultant for Chiron and has received investigator-initiated research grants from Chiron, Pfizer, and Roche Diagnostics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Simbre, V.C., Duffy, S.A., Dadlani, G.H. et al. Cardiotoxicity of Cancer Chemotherapy. Pediatr-Drugs 7, 187–202 (2005). https://doi.org/10.2165/00148581-200507030-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00148581-200507030-00005