Article Text
Abstract
Objective To determine the outcome of neonates with a suspected antenatal diagnosis of congenital heart disease (CHD) who were delivered away from a paediatric cardiothoracic centre and were initially managed in a level 3 Neonatal Intensive Care Unit.
Methods An 18-year ongoing study conducted in a single institution.
Results Between 1992 and 2009, 143 fetuses with suspected CHD were identified, and 124 babies were delivered locally. 13 babies with a normal postnatal echocardiogram and six with isolated arrhythmias were excluded from the study. Structural CHD was confirmed in 105 infants; of these, 94 (90%) survived the neonatal period. Of the 11 neonatal deaths, only four of these infants underwent surgery; most had additional risk factors including: prematurity, very low birth weight, and genetic and other structural congenital anomalies.
Conclusions This study demonstrates that appropriately selected infants with antenatally diagnosed CHD can be safely delivered and initially managed in a non-cardiac centre during their neonatal period. Deliveries need to be carefully planned with close collaboration among neonatologists, obstetricians, paediatric cardiologists, mid-wives and parents.
- Cardiology
- Congenital Abnorm
- Fetal Medicine
- Imaging
- Neonatology
Statistics from Altmetric.com
What is already known on this topic
-
Congenital heart disease can be diagnosed prenatally at the fetal anomaly scan using the 4-chamber view.
-
The Children's Heart Federation has recommended delivery of prenatally diagnosed infants close to cardiac centres.
-
Duct dependent neonatal heart disease is life threatening requiring prostaglandin treatment to maintain ductal patency. Many neonatal cardiac conditions do not need immediate surgical intervention.
What this study adds
-
Neonates with prenatally diagnosed structural heart problems including duct dependent lesions can be safely delivered and initially managed in neonatal intensive care units.
-
Delivery outside a cardiac unit needs a careful postnatal plan with clear communication between the professionals and the parents.
Introduction
Congenital heart disease (CHD) is the most common congenital abnormality in neonates with moderate or severe anomalies occurring in about 6 per 1000 live births.1 It is also an important cause of morbidity and mortality in infancy.2 Prenatal diagnosis of severe cardiac malformations using echocardiography was first reported in 1986.3 Prenatal screening for CHD has been slowly introduced in the UK and with appropriate training of ultrasonographers antenatal detection rates of 2 per 1000 pregnancies can be achieved.4 Identification of the normal 4-chamber view of the fetal heart is now a routine part of the mid-trimester anomaly scan which is offered to pregnant women in the UK between 19 and 22 weeks gestation. As obstetric scanning skills and scanning equipment improve, an increasing number of babies should be diagnosed with CHD prenatally.
Guidelines published by the Children's Heart Federation recommend that ‘if a baby has been antenatally diagnosed with CHD, arrangements for delivery should be made in or near to the paediatric cardiac centre where possible’.5 In practice, this can be difficult because there are only a small number of cardiac centres in the UK and most do not have a colocated delivery unit on site.
We would suggest that a paediatrician with expertise in cardiology can manage these babies safely during the neonatal period in close collaboration with paediatric cardiologists in a nearby cardiac centre. The aim of this study is to assess the neonatal outcome of babies with a suspected antenatal diagnosis of CHD who were delivered and managed in a level 3 Neonatal Intensive Care Unit (NICU) distant to a specialist cardiac centre.
Methods
Data have been collected prospectively over 18 years (1992–2009) to review the outcome of all babies born with suspected CHD. Patients were identified from our local obstetric, neonatal and paediatric cardiac databases. The initial cohort included all babies with suspected CHD diagnosed on the routine fetal anomaly scan performed between 19 and 22 weeks. Ultrasonographers in this institution have been trained to obtain a 4-chamber view as well as outflow tract views of the fetal heart. Nuchal fold thickness measurements were not routinely performed during this period. Confirmation of the suspected antenatal diagnosis was made by paediatric cardiologists specialising in fetal echocardiography. Following this, parents received counselling from the paediatric cardiologist, local obstetrician, neonatologist and cardiac liaison nurse. Plans were made regarding the ongoing pregnancy, mode and place of delivery. The probable postnatal course was also discussed as well as the likely need for early intervention. All babies were scanned soon after birth by a consultant neonatologist with expertise in cardiology to confirm the antenatal diagnosis. Babies diagnosed in this institution but delivered elsewhere were excluded from this study, as were in utero deaths and isolated cardiac arrhythmias.
Results
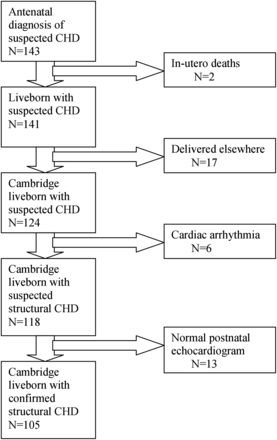
During the study period, 143 fetuses were diagnosed antenatally with suspected CHD. In all, 25 babies were excluded from the study (17 delivered elsewhere, six cardiac arrhythmias and two in utero deaths). Of the 118 babies delivered in Cambridge, 13 had a normal postnatal scan. CHD was confirmed postnatally in 105 babies who were delivered and initially managed in Cambridge (figure 1) and these babies form the main study group.
Patient cohort: outcome following suspected antenatal diagnosis of congenital heart disease.
Of the 105 babies with CHD the median gestational age at birth was 39 (range 26–42) weeks. The male to female ratio was 1.4 : 1. There were 26 (25%) premature births: 26 weeks (1); 28 weeks (1); 29 weeks (2); 31 weeks (5); 32 weeks (2); 33 weeks (2), 34 weeks (3); 35 weeks (3); and 36 weeks (7). Median birth weight was 3100 (596–4589) g, seven babies were very low birth weight (<1500 g). Five infants were twin pregnancies where the co-twin had a structurally normal heart. The mode of delivery was normal spontaneous vaginal delivery 63 (60%) and Caesarean section 42 (40%). Information was not collected on whether labours were spontaneous or induced.
The cardiac lesions in this study group are shown in table 1. Ventricular septal defects (VSDs) (isolated or with coexisting lesions) were the most common anomaly. A total of 41 (41%) babies with duct dependent circulations suspected antenatally and confirmed postnatally were commenced on prostaglandin (PGE1) infusions on admission to NICU. Of these, 21 had left heart and 20 right heart duct dependent lesions. Transfers to the specialist cardiac centre occurred when a cot became available usually within the first few days of life median 2 (range 1–66) days.
Cardiac lesions
Genetic and syndromic abnormalities were identified in 21 (20%) babies (table 2). The cardiac anomalies in the eight babies with Down's syndrome were: atrioventricular septal defect (AVSD) (6), AVSD with tetralogy of Fallot (1) and VSD (1). All patients with Trisomy 18 had VSDs. Extra cardiac anomalies were found in 18 (17%) babies (table 3); of these, four also had chromosomal abnormalities.
Genetic and syndromic diagnoses (n=21)
Associated extra cardiac structural anomalies (n=17)
Overall, 94 (90%) babies survived the neonatal period (28 days). The neonatal death rate for this cohort was 105 per 1000 live births. There were 11 neonatal deaths (table 4). All had confirmed cardiac anomalies with additional problems which were sometimes multiple: four had chromosomal anomalies; three had extra cardiac anomalies; three were premature; and three were very low birth weight. Only four of these infants underwent cardiac surgery; after multidisciplinary discussions among parents, neonatologists and cardiologists, seven infants received palliative care and died on NICU and were never transferred to a cardiac centre. To date, there have been 28 deaths in this cohort; the median age at death was 92 (range 1–800) days. The current overall survival rate following a live birth with confirmed CHD is 73%. Of all the deaths, 5 (18%) had hypoplastic left heart syndrome, 9 (32%) had additional genetic abnormalities, 7 (25%) had other structural problems, 11 (39%) were born prematurely, and some problems coexisted.
Details of neonatal deaths (n=11)
There were 13 ‘false positive’ diagnoses in the overall cohort who had structurally normal postnatal echocardiograms. Importantly, two had karyotype anomalies, and three had significant cardiac asymmetry associated with an omphalocoele, Morgani diaphragmatic hernia and large hepatic arteriovenous malformation. In six pregnancies, an antenatal diagnosis of coarctation had been suspected due to ventricular asymmetry and great artery size discrepancy. Postnatal scans at birth and during the first year of life were all normal. In one case, a suspected VSD was not confirmed postnatally.
Discussion
After prematurity, cardiac abnormalities are the leading causes of death in the neonatal period. CHD is not always easily diagnosed in the early postnatal period as many babies can be completely asymptomatic and have no obvious clinical signs.6 Antenatal scans may help identify fetuses with CHD which are often at the severe end of the spectrum. Approximately 25% of all cases of CHD can be diagnosed prenatally.7 Fetal diagnosis may help with planning optimal postnatal management and also allows time for the parents to be informed and counselled appropriately. They can then decide on whether they wish to continue with the pregnancy or opt for termination.8 The termination rate varies widely in different studies.9 ,10 Most of these studies have been conducted by specialist fetal cardiac centres scanning pregnant mothers who are at a higher risk of having a baby with heart disease. The mothers in this study were considered to come from a low risk population.
It is well recognised that certain chromosomal and extra cardiac anomalies can be strongly associated with cardiac lesions.11 It is important, therefore, for all confirmed cases of CHD to undergo a thorough fetal assessment to detect coexistent abnormalities and for mothers to be offered karyotype analysis including 22Q11 studies. The recognition of a specific syndrome or diagnosis is important for the overall management of the baby and for the rest of family members.12 In this study chromosomal abnormalities were found in 20% of babies, which is in accordance with other studies.10 ,11 ,13 ,14
The Children's Heart federation has published recommendations on the standards of care expected for children with CHD. In cases of antenatal diagnosis, it recommends that ‘close liaison is established between district general hospital, obstetric ultrasonography and the cardiac centre’ before birth and ‘arrangements for the delivery should be made in or near to the paediatric cardiac centre, where possible’.5 However, there are a small number of cardiac units across the UK and most of these units do not have colocated delivery units. Recent fetal cardiology standards published by the British Congenital Cardiac Association15 and a review of fetal cardiac screening16 highlight the option of local delivery after prenatal diagnosis provided a postnatal management plan has been formulated with the cardiac unit. This study confirms that local delivery is safe and effective with careful planning. While we aim for local delivery, policies have changed over time with new evidence.17 Initially, babies diagnosed with transposition of the great arteries (TGA) were delivered in Cambridge. We have recently arranged for two babies with TGA to be delivered in a unit adjacent to the cardiac centre in case an urgent balloon septostomy was required. Similar arrangements would be considered for babies with left heart problems with an intact atrial septum.
Local delivery has major advantages for the family, reducing travel times, social upheaval and increases the chance of normal delivery. Importantly, planned local delivery avoids unnecessary admission and occupancy of specialist cardiac beds. Our patient cohort included babies with complex and non-complex lesions. Transfer may not always be necessary during the neonatal period especially if the cardiac lesion is not duct dependent and the baby is stable and does not require immediate surgery. These babies may need careful observation which can be safely undertaken by paediatricians in local units in liaison with specialist cardiologists. A significant proportion (40%) of this cohort had duct dependent lesions and were commenced on PGE1 infusions immediately after birth and transferred to the cardiac centre when a bed became available. Most were moved during the second day of life. One baby with pulmonary atresia born 10 weeks early weighing 985 g was maintained on PGE1 for 66 days to grow before being transferred for a Blalock Taussig shunt.18 Babies commenced on PGE1 were not routinely intubated and ventilated unless it was clinically indicated. Many of our patients required only a short stay in the cardiac unit following surgery or catheter interventional procedures. Most were discharged home but some were subsequently returned to us for continuing care after intervention, most often to establish feeding. All patients had out-patient follow-up in the local specialist cardiac outreach clinic. We demonstrated a neonatal death rate of 10% in our cohort, which is considered low. Looking at other studies the neonatal death rates can vary considerably.10 ,11 ,13 ,14 This may reflect differences among the populations studied as well as changes in the management of CHD over the last decade.
This study shows that infants with prenatally diagnosed CHD can be safely delivered and initially managed at a level 3 neonatal unit. It is important to stress that this study refers to the experience of a single level 3 neonatal unit where the care of infants with CHD is largely coordinated by one consultant with expertise in cardiology. Close liaison with cardiologists and careful communication with parents, obstetricians, mid-wives and paediatricians regarding postnatal management is essential. An agreed postnatal plan is documented in maternal notes and ‘prebirth’ notes created for the baby and in letters to obstetricians and neonatologists, which are also copied to parents. The importance of careful audit cannot be overemphasised to determine the accuracy of antenatal diagnosis. There should also be a careful review of false positive and negative screening scans. This model may not be applicable for in all units across the UK but if carefully developed there are major benefits for the parents, their babies and specialist cardiac centres. It also ensures that paediatric departments do not become deskilled in the management of infants with CHD.
References
Footnotes
-
Contributors LM was one of the obstetric ultrasonographers. She was the coordinator for information collected from fetal medicine and maintained the fetal records. WK is the Paediatric cardiologist for Cambridge and coordinates postnatal and ongoing paediatric care. He is responsible for paediatric database. He was instrumental in developing and driving this project forward. He has coordinated completion of the paper. KA pulled together all the data and analysed patient demographics and provided the first draft of the paper. RY is the specialist cardiologist who performs specialist fetal echocardiograms to confirm the antenatal diagnosis. RY and WK agree on the postnatal management plan and provide long term follow-up of all patients.
-
Competing interests There are no conflicting interests. This work has not been submitted for publication in any other journal.
-
Provenance and peer review Not commissioned; externally peer reviewed.