Article Text
Abstract
Positron emission tomography, cardiovascular magnetic resonance and multislice computed tomography have contributed to changing our pathophysiological understanding of many conditions. Clinically, they have provided new tools for the identification of preclinical disease and a better understanding of how disease progresses. The application of these imaging modalities to preclinical disease and the use of these techniques in patients with overt cardiovascular disease are reviewed.
- CAC, coronary artery calcium
- CAD, coronary artery disease
- CMR, cardiovascular magnetic resonance
- CT, computed tomography
- FDG, fluorodeoxyglucose
- MRI, magnetic resonance imaging
- MSCT, multislice computed tomography
- PET, positron emission tomography
- SPECT, single photon emission computed tomography
Statistics from Altmetric.com
- CAC, coronary artery calcium
- CAD, coronary artery disease
- CMR, cardiovascular magnetic resonance
- CT, computed tomography
- FDG, fluorodeoxyglucose
- MRI, magnetic resonance imaging
- MSCT, multislice computed tomography
- PET, positron emission tomography
- SPECT, single photon emission computed tomography
The constant development and refinement of non-invasive cardiac imaging over the past two decades have contributed to changing our pathophysiological understanding of many conditions. Clinically, these developments have provided new tools for the identification of preclinical disease and a better understanding of how disease evolves and reaches its terminal stage. The focus of this review is to summarise how positron emission tomography (PET), cardiovascular magnetic resonance (CMR) and multislice computed tomography (MSCT) have contributed to this process and how their combination (fusion) can further revolutionise cardiology. Obviously, other well-established techniques such as echocardiography and single photon emission computed tomography (SPECT) will continue to be essential in clinical practice for disease diagnosis and stratification of patients, whereas these more expensive and less available techniques will provide clinicians with new tools for the exploration of very early and very late phases of cardiac diseases. Accordingly, this article is divided into two parts: the application of these imaging modalities to preclinical disease and the use of these techniques in patients with overt cardiovascular disease.
PRECLINICAL CORONARY ARTERY DISEASE AND MICROVASCULAR DYSFUNCTION
Positron emission tomography
By applying mathematical modelling to tracer kinetics derived from time–activity data, PET permits non-invasive quantification of regional myocardial blood flow. Water labelled with oxygen-15 and ammonia labelled with nitrogen-13 have been validated in animal studies against radioactive microspheres and provide comparable results.1,2 The generator-produced cation rubidium-82 has also been used, although it provides inferior data due to its reduced extraction fraction at higher flows and its very high positron track, which degrades the scanner’s resolution.3
Measurement of absolute myocardial blood flow (in ml/min/g) and coronary flow reserve provides information on both the macrocirculation (that is, epicardial coronary arteries) and the microcirculation (vessels from < 3 to 400 μm in diameter). In the absence of detectable coronary stenoses, abnormal coronary flow reserve is a marker of early alterations of vascular reactivity.4,5 Coronary endothelial and microcirculatory dysfunction have been shown in patients with coronary risk factors such as hypercholesterolaemia, essential hypertension, diabetes mellitus and smoking by a variety of endothelial-independent or endothelial-dependent stimuli.6–,8 In other words, assessment of absolute myocardial blood flow and coronary flow reserve provides a way to document how risk factors translate into measurable damage to the coronary circulation. A most attractive application appears to be the monitoring of risk factor modification by PET perfusion measurement as a surrogate end point.9,10
So far only a few studies have assessed the prognostic value of abnormal myocardial blood flow and coronary flow reserve. Impaired vasodilator capacity has been shown to have significant prognostic value in patients with both dilated11,12 and hypertrophic cardiomyopathy.13
Computed tomography
The value of computed tomography (CT) in the detection of subclinical atherosclerosis is its assessment of coronary artery calcium (CAC). Both electron beam CT and MSCT can be used to assess CAC. MSCT also allows non-invasive coronary angiography, which is probably more important in advanced coronary artery disease (CAD; see below).14,15 The frequency and extent of CAC increase with age16 and are higher in men than in women.14 Still, the presence of calcifications in an epicardial coronary artery should not be considered normal and reflects early atherosclerosis. The extent of CAC is a marker that is highly correlated with atherosclerotic disease burden14,17 but is not indicative of obstructive CAD.14,15,17 The so-called Agatston score has been developed to quantify the presence and extent of CAC; for those patients with high-risk scores, significant CAD is often present. Specifically, for patients with moderate- to high-risk CAC scores (that is, scores ⩾ 100 and ⩾ 400), the prevalence of obstructive CAD increases significantly.17–,19 Conversely, a negative or low-risk study has a high negative predictive value (about 95%) and virtually excludes significant CAD.18,19
Besides diagnosis, CAC is useful for prognostic purposes. Several large studies (with > 1000 patients) evaluated selected high-risk but asymptomatic populations for their risk of cardiovascular disease.16,20–,24 Figure 1⇓ provides a synopsis of available evidence for expected 10-year mortality or rate of myocardial infarction based on several large published series from Greenland et al22 and Shaw et al,16 as well as a recent meta-analysis.24 This figure shows that the relative risk of death or CAD events increases linearly with the extent of CAC. High-risk CAC scores > 400 are associated with a 10.0-fold to 12.3-fold higher risk of death or CAD events.
Relative risk of all-cause death, cardiovascular death or myocardial infarction, or overall cardiac events from two large observational cohorts (Shaw et al16 and Greenland et al22) and a meta-analysis (Pletcher et al24). CAC, coronary artery calcium.
More recent data were derived from the St Francis Heart Study,25 a prospective, population-based study of 4903 asymptomatic patients aged 50–70 years who underwent electron beam CT to assess CAC. Follow up was obtained in 4613 (94%) participants at 4.3 years and 119 had an event related to atherosclerotic cardiovascular disease. Patients with events had a significantly higher CAC score than those without events. Moreover, the relative risk for an event in the presence of a CAC score ⩾ 100 was 9.6. Most important, CAC score predicted events independently of standard risk factors and was superior to the Framingham risk score.
When patients with a raised CAC score were treated with statins and vitamins C and E, however, their CAC score was not reduced over time, indicating that, although the CAC score can identify patients at increased risk, the therapeutic implications of a raised CAC score are not yet established.26
Cardiovascular magnetic resonance
The strength of CMR in detecting subclinical atherosclerosis is in plaque imaging, at present predominantly applied to large vessels. Initial studies in animal models showed the feasibility of evaluating aortic atherosclerotic plaques. Skinner et al27 illustrated the use of CMR for atherosclerotic lesions in the abdominal aorta of rabbits. In serial CMR studies, the authors showed the feasibility of evaluating progression of disease. Moreover, some plaque components (for example, the fibrous cap and necrotic core) could be identified and correlated well with histological and gross examination.
Toussaint and colleagues28 subsequently applied CMR plaque imaging in six patients who required surgical carotid endarterectomy. Comparison of the magnetic resonance images with histological analyses confirmed the feasibility of discriminating fibrous caps, lipid cores and calcifications in human atheromatous lesions.
Following these observational and comparative studies, Corti et al29 used CMR plaque imaging to assess the response to treatment. In 18 asymptomatic, hypercholesterolaemic patients with documented aortic or carotid plaques, serial CMR studies showed a significant reduction in atherosclerotic burden (expressed as vessel wall thickness and vessel area) after 12 months of treatment with statin.
The next step, currently being evaluated, is the use of intravascular coils with CMR. This approach substantially improved the resolution of vessel wall imaging and allows more subtle evaluation of plaque components including lipid, collagen, calcium and thrombus formation. Ex vivo imaging of the aorta had sensitivity and specificity of 83% and 100%, respectively, for detection of fibrous cap and necrotic core.30 Substantial data from human studies with intravascular magnetic resonance imaging (MRI) are not yet available.
IMAGING VULNERABLE PLAQUES
Atherosclerotic lesions develop in the course of a specific inflammatory process triggered by an initial injury of the endothelium and may result in mechanical destabilisation and rupture of the atherosclerotic plaque, potentially leading to thrombosis and vessel occlusion with life threatening clinical sequelae.31,32 Despite primary and secondary prevention, such events still account for one third of all deaths worldwide and constitute a major source of disability and healthcare costs. Therefore, identifying individual patients with a high risk of plaque rupture is an important challenge in clinical medicine. Several non-invasive techniques including CMR and MSCT are being tested for their applicability to identifying such patients by assessing morphological criteria associated with high risk of atherosclerotic plaque rupture.
In contrast, non-invasive scintigraphic techniques such as PET make use of radiolabelled molecules (radioligands) designed to specifically target individual inflammatory activities involved in the development of atherosclerotic plaques.33 Plaque rupture is usually a consequence of inflammatory cell activity within the plaque. Techniques that visualise plaque anatomy and composition do not provide information on plaque inflammation. Fluorodeoxyglucose (FDG) labelled with fluorine-18 with PET has been used to image inflammatory cell activity. In a study in patients with symptomatic carotid atherosclerosis, FDG with PET (co-registered with CT) was used to identify inflammation within plaques.34 The estimated net FDG accumulation rate in symptomatic lesions was 27% higher than in contralateral asymptomatic lesions, whereas there was no measurable FDG uptake into normal carotid arteries.
A variety of cellular molecular targets involved in the progression and potential rupture of vulnerable plaques have been identified: macrophage density, apoptosis, protease activity and many others.33 Different PET-based approaches to image these targets are being developed and may help identify patients with a future high risk of plaque rupture, especially in combination with high-resolution morphological imaging of the coronary arteries by PET-CT.
OVERT CARDIOVASCULAR DISEASE
Overt disease comprises the entire spectrum from diagnosis and prognosis in CAD to evaluation of ischaemic cardiomyopathy. As pointed out before, echocardiography and SPECT remain the most important techniques for diagnostic and prognostic purposes. CMR can also be used for diagnosis of CAD; both dobutamine CMR (assessing ischaemia by looking at inducible wall motion abnormalities) and stress–rest perfusion CMR (assessing ischaemia by looking at inducible perfusion abnormalities) have been reported in the literature for diagnosis of CAD.35
PET stress–rest perfusion imaging (mainly with 82Rb- and 13N-labelled ammonia) has also been used for diagnosis and prognosis in CAD. On the basis of pooled analysis of seven studies with 633 patients, the sensitivity and specificity of PET for detection of CAD were 89% and 86%, respectively36; the high specificity is (at least partially) related to attenuation correction. A potential advantage of perfusion imaging with CMR or PET is the high spatial resolution of these techniques making differentiation between epicardial and endocardial perfusion possible. PET imaging is also useful for determining prognosis. Patients with a normal PET perfusion scan had an excellent prognosis, whereas patients with extensive abnormalities had a 76% event-free survival.37
CMR AND MSCT: NON-INVASIVE ANGIOGRAPHY
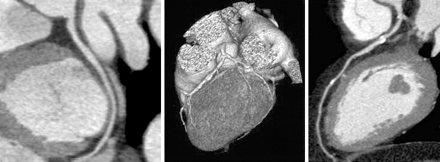
Although cardiac catheterisation allows diagnosis of significant CAD with excellent spatial (0.2 mm) and temporal resolution (5 ms), the technique still has several drawbacks including its high cost and invasive nature. Over the past few years, CMR and MSCT have been rapidly developed for non-invasive imaging of the coronary arteries. In 1993, Manning et al38 reported the first results of non-invasive coronary angiography with MRI with sensitivity and specificity of 90% and 92%, respectively. Additional reports varied significantly with sensitivities ranging from 38–88% and specificities ranging from 72–100%.39 Of note, on average 25% of segments are of non-diagnostic quality. It is anticipated that results will further improve with 3 T CMR. Regarding MSCT, the initial studies were performed with four-slice scanners and pooled data of 11 studies yielded a mean sensitivity of 80% with a specificity of 94%.39 The percentage of non-assessable segments was on average 22%. Introduction of 16-slice scanners has led to increased accuracy: pooled data of 11 studies yielded mean sensitivity and specificity of 88% and 96%, respectively; on average, 4% of segments were of non-diagnostic quality.39 The consistently high specificities and negative predictive values that have been reported thus far underline the potential of the technique to exclude CAD. In particular, the specificity of MSCT appears significantly higher than that of MRI.39 In addition, further improvement in diagnostic performance is expected with the 64-slice scanners. Accordingly, MSCT may be the preferred technique for non-invasive angiography.39 Figure 2⇓ presents some examples from patients of non-invasive angiography with MSCT. The major drawbacks of the technique are the radiation burden, which is around 10 mSv, and the need for low heart rate during MSCT (obtained by administration of β blockers, which is not feasible in all patients). Meta-analysis of the available MSCT and MRI angiographic studies showed a significantly higher specificity for MSCT39; however, the techniques are constantly under development, which limits meta-analysis of data. An important topic of uncertainty is which patient populations may benefit most from non-invasive angiography. Results from the aforementioned meta-analysis39 showed that almost 80% of patients had CAD, illustrating the high prevalence of CAD in the available studies. In the clinical setting, however, non-invasive angiography may be more useful in patients with an intermediate likelihood of CAD.
Examples from patients of multislice computed tomography MSCT non-invasive coronary angiography. (Left) Normal left circumflex coronary artery (curved multiplanar reconstruction). (Middle) Three-dimensional volume-rendered reconstruction. (Right) Curved multiplanar reconstruction of the left anterior descending coronary artery, showing an intermediate stenosis proximal with calcifications (white spots).
PET AND CMR: ASSESSMENT OF VIABILITY AND SCAR TISSUE
Glucose utilisation has been assessed extensively by FDG PET to identify dysfunctional but viable myocardium and to predict improvement in left ventricular function after revascularisation.40,41 Pooled analysis of 20 studies with 598 patients showed a sensitivity of 93% to predict recovery of function, which was higher than that of SPECT imaging with agents labelled with thallium-201 or technetium-99 m or that of dobutamine stress echocardiography.42 According to the American College of Cardiology/American Heart Association/American Society of Nuclear Cardiology guidelines, FDG PET is considered a class I indication for assessment of viability in ischaemic cardiomyopathy.36 FDG can be used in combination with a perfusion tracer, making distinction between (repetitive) stunning, hibernation and (non-)transmural scar tissue possible.43 Alternatively, FDG can be used alone during hyperinsulinaemic euglycaemic clamping, allowing for absolute quantification of regional myocardial glucose utilisation.44 This approach may have the highest sensitivity to detect viable myocardium.
Most studies have focused on prediction of recovery, but this may not be the ideal end point. Improvement in heart failure symptoms, prevention of ongoing left ventricular remodelling and long-term prognosis may be more important clinically. Studies on long-term outcome have consistently shown that viability on FDG PET was associated with a high event rate if patients were treated medically, but also that delayed revascularisation resulted in worse outcome.41
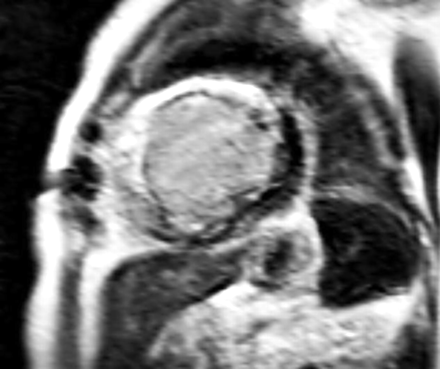
Delayed contrast-enhanced imaging with CMR has become very popular to evaluate patients with ischaemic cardiomyopathy.45 The contrast accumulates in infarcted tissue, and animal experiments have shown an excellent agreement between contrast-enhanced CMR and the histological extent of infarction. In particular, the high spatial resolution of CMR allows precise delineation between transmural and subendocardial scar tissue (figs 3⇓ and 4⇓). Kim and colleagues46 evaluated 50 patients undergoing revascularisation and reported that the transmurality of the infarcted area was inversely related to the likelihood of recovery after revascularisation. From a clinical perspective, it is important to realise that contrast-enhanced CMR reflects scar tissue, whereas FDG PET can identify the amount of residual viable myocardium in jeopardised segments. It would therefore be of interest to evaluate the integration of information from both modalities for prediction of functional recovery after revascularisation.
Contrast-enhanced cardiovascular magnetic resonance study of transmural scar tissue in the anteroseptal wall (white, hyperenhanced).
Contrast-enhanced cardiovascular magnetic resonance study showing non-transmural scar in the anterolateral region and a small amount of scar tissue inferiorly (white, hyperenhanced).
CLINICAL APPLICATIONS OF PET AND CT IN CARDIOLOGY AND FUTURE PERSPECTIVES
To optimally evaluate the haemodynamic significance of coronary artery lesions (to guide treatment), anatomical information must be integrated with the haemodynamic consequences (does the lesion result in ischaemia?). PET/CT scanners have been introduced worldwide, making integration of these two parameters possible: angiography with the CT portion and stress–rest perfusion measurements with the PET portion of the system.
Preliminary results are encouraging. In a recent study, the concept was evaluated by using an early PET/CT scanner with a four-slice, 0.5 s rotation CT system with 13N-labelled ammonia as a flow tracer. Twenty-five patients with suspected CAD underwent PET/CT, yielding a sensitivity and specificity of 90% and 98%, respectively, to detect haemodynamically significant stenoses (as compared with the combination of stress–rest PET perfusion imaging and invasive coronary angiography).47 With the ongoing development of PET/CT systems, integration of 64-slice scanners and PET perfusion with 82Rb will reduce data acquisition, enhance resolution and improve diagnostic performance. To further increase patient throughput and meet the high demand rate for patient studies, it will be important to explore integration of SPECT cameras with high-end CT systems. As probably 90% of all questions about myocardial perfusion can be solved with SPECT perfusion imaging, for which radiopharmaceuticals are widely available, this is an interesting alternative to PET/CT. As all the technologies are forthcoming, the next five years should clarify whether integrated procedures with CT and nuclear medicine techniques make clinical sense and are economically viable.
TECHNICAL ASPECTS AND FUTURE DEVELOPMENT
Although PET has the potential to image and measure pathophysiological and molecular processes in vivo, its position in cardiovascular research and patient care has primarily been based on its capacity to image perfusion and glucose metabolism. To achieve its full potential, some major challenges have to be addressed.
Quantification
Since the late 1970s, spatial resolution of PET scanners has steadily improved, and there is scope for further improvement. With the improved resolution, the impact of patient movement on quantification becomes more critical, at least if the improved resolution is used to its full potential (for example, voxel-by-voxel analyses). This highlights the need for methods that can correct for patient movement, not only within one scan (such as between frames within a dynamic emission scan) but also between scans (such as between transmission and emission scans). Also related to the improvement of spatial resolution is the need to correct for cardiac and respiratory motion. This can be achieved by gating techniques, especially when the scanner is equipped with list mode acquisition hardware and software. Although this is feasible for static scans, its use for dynamic scans has not been investigated, as it compromises statistical quality of the acquired data.
Lastly, many new scanners, in particular PET/CT scanners, are three-dimensional only scanners. These scanners are more sensitive than two-dimensional scanners (better image quality), but the contribution of scatter is also much higher. As a result, more sophisticated scatter correction techniques are needed, in particular to deal with activity from outside the field of view of the scanner (for example, hepatic activity). In addition, attenuation correction methods (singles source, CT scan) need to be validated for quantitative purposes.
Molecular imaging
PET permits imaging and quantification of molecular interactions and pathways with picomolar sensitivity. As most radioligands are metabolised in the human body, development of tracer kinetic models also requires that the presence of labelled metabolites be taken into account. For this purpose combination with magnetic resonance spectroscopy could be very useful.
Many processes can be studied—for example, receptor density, enzyme activity, inflammatory processes and gene expression. In particular, several positron-labelled tracers for imaging cardiac sympathetic and parasympathetic agonists and receptors with PET have been developed and validated. These include the catecholamine analogue hydroxyephedrine labelled with carbon-11, 11C-CGP 12177 for the measurement of β adrenoceptor densities, and 11C-MQNB for the measurement of muscarinic receptors.48 Use of these ligands alone or in combination (for example, 11C-hydroxyephedrine and 11C-CGP 12177) has provided evidence of abnormal autonomic function in different cardiac diseases including dilated and hypertrophic cardiomyopathy and in idiopathic arrhythmogenic diseases such as Brugada’s syndrome.49,50 Importantly, the prognostic value of β adrenoceptor downregulation measured soon after acute myocardial infarction was recently shown by using PET with 11C-CGP 12177.51
Clinical applicability
Research studies are needed to gain new insights in pathophysiology and to assess the efficacy of treatment. For maximal clinical impact, however, it is important to derive simplified protocols that are calibrated against the more complicated research protocols. A potential danger is that simplified methods may be introduced before proper validation.
Arterial sampling is less suitable for routine clinical studies. The use of image-derived input functions should therefore be further evaluated. For example, the ascending aorta apparently is the optimal structure for defining image-derived input functions in case of FDG, whereas the left atrium and ventricle are equally suitable in case of water studies. In both cases, however, these regions have to be defined by hand with the inherent risk of interobserver variation. Development of automatic procedures (for example, cluster analysis) should have high priority.
To reduce interobserver variability even further, automatic methods for defining myocardial regions of interest should also be developed. Automatic definition of regions of interest is important not only for clinical studies (being less time consuming) but also for multicentre trials (for standardisation). PET data often are analysed by region of interest, producing results that do not make optimal use of the resolution of the scanner, and heterogeneity within those regions of interest may be overlooked. Methods are needed to derive parametric images—that is, representation of results on a voxel-by-voxel basis. Initial efforts have been reported, but this clearly is an area that needs further research.
FUSION OF THE IMAGING MODALITIES IS THE FUTURE: THE CHALLENGE OF COREGISTRATION
From a computational perspective, one of the major benefits of adopting a hardware-based approach for image fusion is that it permits the acquisition of co-registered anatomical and functional images. Traditionally, this is achieved at a software level through the use of landmark-, surface- or intensity-based co-registration techniques. Landmark methods typically are based on either external fiducial or anatomical landmarks, whereas surface- and intensity-based techniques explore the intrinsic information content of different image features. Despite significant advances in software-based co-registration techniques, issues of accuracy and user interaction have limited their routine clinical use on a patient-by-patient basis. The real benefit of fusing different imaging modalities, however, is in the ability to use the anatomical information acquired in situ to improve the scan efficiency and resolve complex physiological motion for enhancing the resolution details of the functional image. In the case of PET/CT, for example, the CT images can be used to correct attenuation of the PET emission data, thus eliminating the need for a separate and often lengthy PET transmission scan. In addition to increasing the overall scan efficiency, the method provides effectively noiseless attenuation correction factors that cannot be achieved by the current approaches. Before the technique becomes clinically useful, however, several technical hurdles must be overcome. These include spatial mismatches arising from patient motion (particularly respiratory-induced visceral deformation), errors in the attenuation map due to the use of contrast agents or the presence of metal objects in the patient during the CT scan, truncation of the CT field of view and beam-hardening artefacts.52,53 Among these, the most challenging issue is the effective handling of respiratory motion because of its poor intercycle consistency. For PET/CT, this effect is further amplified because CT is normally acquired with breath hold on full inspiration, whereas the PET is acquired during free breathing. Although cardiac motion due to respiration acts predominantly in the craniocaudal direction, supplementary translational motion in the other two directions is also significant. Furthermore, the heart rotates to a degree that varies from person to person. Non-rigid deformation also varies in degree between patients and, when present, is most significant in the left ventricular apex, the right atrium and the region of the right coronary artery. Owing to the nature of this complex, non-rigid deformation, effective motion modelling is required to predict the extent of respiratory-induced motion in a subject-specific manner.54 This is expected to be an area of active development in future years, as in the case of PET/CT the benefits of low statistical noise and rapid transmission imaging far outweigh the potential problems listed above.
CONCLUSIONS
The role of PET has been established in clinical evaluation of perfusion and metabolism in patients with CAD. It will continue to have a role in research particularly in molecular imaging. MRI and MSCT are also emerging as technologies with clinical application. Preclinical definition of disease is becoming an important focus as early interventions attempt to prevent the progression to established disease. All these advanced modalities provide unique and complementary information on pathophysiology and anatomy. Optimal use will require consideration of this complementary nature, facilitated by fusion or hybrid techniques. Continued research to optimise these technologies and to define their clinical role remains a priority for improving the diagnosis of cardiovascular disease and the therapeutic evaluation of patients.
REFERENCES
Footnotes
Published Online First 30 December 2005