Article Text
Abstract
OBJECTIVE To characterise the central and regional haemodynamic effects of insulin in patients with chronic heart failure.
DESIGN Single blind, placebo controlled study.
SETTING University teaching hospital.
PATIENTS Ten patients with stable chronic heart failure.
INTERVENTIONS Hyperinsulinaemic euglycaemic clamp and non-invasive haemodynamic measurements.
MAIN OUTCOME MEASURES Change in resting heart rate, blood pressure, cardiac output, and regional splanchnic and skeletal muscle blood flow.
RESULTS Insulin infusion led to a dose dependent increase in skeletal muscle blood flow of 0.36 (0.13) and 0.73 (0.14) ml/dl/min during low and high dose insulin infusions (p < 0.05 and p < 0.005 v placebo, respectively). Low and high dose insulin infusions led to a fall in heart rate of 4.6 (1.4) and 5.1 (1.3) beats/min (p < 0.05 and p < 0.005 v placebo, respectively) and a modest increase in cardiac output. There was no significant change in superior mesenteric artery blood flow.
CONCLUSION In patients with chronic heart failure insulin is a selective skeletal muscle vasodilator that leads to increased muscle perfusion primarily through redistribution of regional blood flow rather than by increased cardiac output. These results provide a rational haemodynamic explanation for the apparent beneficial effects of insulin infusion in the setting of heart failure.
- blood flow
- heart failure
- insulin
- muscle
Statistics from Altmetric.com
Chronic heart failure is characterised by cardiac dysfunction, abnormalities of the peripheral circulation, and activation of neurohumoral mechanisms. There is likely to be a complex dialogue between these systems that ultimately leads to the clinical syndrome of heart failure. It has been suggested that insulin resistance may also be important in the pathophysiology of heart failure.1 ,2
Insulin resistance is usually shown by diminished hypoglycaemic action of insulin and it is believed that abnormalities of glucose handling at the cellular level account for its deleterious effects in cardiovascular diseases. These abnormalities may be particularly important in cardiac and skeletal muscle of patients with heart failure.
Insulin has other properties that may be important. It has been shown to cause peripheral arteriolar vasodilatation in normal healthy subjects.3 This effect is attenuated in patients with type II diabetes and hypertension.4 ,5 It has been suggested that the failure of insulin mediated vasodilatation is in part responsible for the insulin resistance associated with these conditions. Little is known, however, about the aetiology of insulin resistance or about the integrity of insulin mediated vasodilatation in chronic heart failure.
Peripheral blood flow is redistributed in heart failure: blood flow to the coronary and cerebral circulations is preserved at the expense of blood flow to other tissues. Reduced skeletal muscle blood flow is a major determinant of exercise tolerance and functional impairment in heart failure.6 ,7 Blood flow to other vascular beds such as the gut is less important. The mechanism of action of any agent that can reverse this abnormal redistribution of blood flow is therefore of considerable interest. Measurement of regional blood flow is therefore useful in assessing mechanisms that control the circulation in heart failure and the effects of treatment.
The purpose of this study was to evaluate the central and regional haemodynamic effects of insulin in a group of patients with chronic heart failure.
Patients and methods
PATIENTS
Ten male patients aged 54–82 (mean 69) years with chronic heart failure were studied. All had mild to moderate heart failure (New York Heart Association functional class II–III) and were ambulatory outpatients. Heart failure was caused by ischaemic heart disease in six patients and dilated cardiomyopathy in the remaining four. All had an ejection fraction measured echocardiographically of < 45% or left ventricular end diastolic diameter of > 6 cm. All were taking diuretics and a mean daily dose of furosemide of 80 mg (range 40–160 mg). Nine were taking angiotensin converting enzyme (ACE) inhibitors and the 10th patient was taking a long acting nitrate but no patients were being treated with β blockers. All had been taking stable medication for at least four weeks. No patient was taking any treatment that affects glucose metabolism and none had another condition known to affect insulin sensitivity. All patients had normal fasting glucose (range 3.1–5.7 mmol/l) and glycosylated haemoglobin (range 4.8–5.9%) within the normal, non-diabetic range for the hospital.
METHODS
The patients were studied on two occasions in a temperature controlled laboratory (23–24°C) at 8 am after an overnight fast and having omitted their usual drugs that morning. One day served as a control day and the other was the test day; the order of these was randomised.
HYPERINSULINAEMIC EUGLYCAEMIC CLAMP
Patients were studied in the supine position after 30 minutes' rest. The left hand was placed in a chamber with air circulating at 50–60°C to arterialise the venous blood and an 18 gauge cannula was inserted retrogradely into a vein on the dorsum of the hand. A second 18 gauge cannula was inserted anterogradely into a vein in the antecubital fossa of the same arm for infusion of insulin and glucose. Following a priming infusion, soluble insulin (Human Actrapid, Novo Nordisk, Crawley, UK) was infused at a rate of 40 mU/m2/min for 90 minutes and then increased to 100 mU/m2/min for a further 90 minutes. These doses of insulin were calculated to produce a steady state plasma insulin concentration of approximately 100 and 250 mU/l, respectively. Blood sugar concentration was measured every five minutes and 20% glucose was infused at a variable rate to maintain blood sugar concentrations at the fasting, baseline concentration. This method of hyperinsulinaemic euglycaemic clamp has been described in detail elsewhere.8 On the control day the procedure was similar but normal saline was infused and repeated haemodynamic measurements were made for 90 minutes only.
HAEMODYNAMIC MEASUREMENTS
Haemodynamic variables were measured at rest at baseline and after 30 and 60 minutes of the first dose of insulin and then after 30 and 60 minutes of the second dose. On the control day haemodynamic measurements were made after 30 and 60 minutes of the placebo infusion.
Heart rate was monitored continuously and blood pressure measured by auscultation in the right arm.
Cardiac output
Cardiac output was measured non-invasively using the indirect Fick principle of monitoring respiratory gases with a mass spectrometer. Carbon dioxide was used as the indicator. Carbon dioxide production is calculated from minute ventilation and mixed expired carbon dioxide concentration. The partial pressure of carbon dioxide in pulmonary venous (systemic arterial) blood is derived from end tidal carbon dioxide concentration and the partial pressure in mixed venous blood is obtained following a rebreathing manoeuvre. These three variables can then be used to solve the Fick equation. The technique has been validated against thermodilution.9
Forearm blood flow
Forearm blood flow was measured in the right arm by venous occlusion plethysmography using mercury in Silastic strain gauges. The coefficient of variation for this technique in our laboratory is 11.5%.10
Superior mesenteric artery blood flow
Superior mesenteric artery flow was measured by transcutaneous Doppler ultrasound using a Diasonics Prisma system (Diasonics International, Les Ulis, France). The vessel was identified by ultrasound and the sample volume of the pulsed Doppler system was adjusted to the size of the vessel being interrogated. Doppler spectral analysis was recorded with the patients' breath held in midinspiration. Mean velocity was calculated from at least 10 Doppler waveform complexes. Vessel diameter was calculated from a mean of four values. Measurements and Doppler signals were recorded from the proximal portion of each vessel. Blood flow was calculated by the machine's in-built software according to the following equation:
π (vessel diameter/2)2 × mean velocity
The coefficient of variation of this technique in our laboratory is < 6%.11
Vascular resistance
Vascular resistance in arbitrary units for the superior mesenteric and forearm vascular beds was calculated using the formula: vascular resistance = (mean arterial pressure/blood flow) × 100.
Biochemical measurements
Arterialised blood was sampled at baseline and every 30 minutes during the experiment for measurement of plasma insulin and catecholamines. Blood was collected and allowed to clot in plain tubes before being spun at 2000 rpm in a centrifuge for 20 minutes. The supernatant was stored in plain tubes at −80ºC for subsequent analysis. Insulin was measured by solid phase radioimmunoassay (Diagnostic Products Corp, Los Angeles, USA). In our laboratory this method has an intra-assay coefficient of variation of 7.0% and interassay coefficient of variation of 10.4%. Plasma noradrenaline (norepinephrine) was measured by high performance liquid chromatography with electrochemical detection after extraction. The intra-assay and interassay coefficients of variation were 6.2% and 8.5%, respectively.
STATISTICAL ANALYSIS
All results are expressed as mean (SD). Baseline values for haemodynamic variables on each visit were compared by pairedt tests. To examine the effect of the insulin infusion on haemodynamic variables, changes from baseline were calculated for measurements at each time point. Analysis of variance for repeated measures was calculated using the three insulin doses (placebo, low dose, and high dose) as a within subject factor. Where analysis of variance suggested a significant effect of the insulin infusion, post hoc paired t tests were done to compare each insulin dose with placebo. A probability value of p < 0.05 was considered significant.
ETHICAL STANDARDS
The local ethics committee approved the study and all patients gave written, informed consent.
Results
There were no significant differences in any of the haemodynamic or metabolic variables on the two study days. The mean baseline plasma insulin concentration was 14 (1) mU/l on the control day and 14 (3) mU/l on the study day. Mean insulin concentration during the placebo infusion was 19 (2) mU/l. The mean plasma concentration of insulin rose to 103 (5) mU/l during the infusion of 40 mU/m2/min and 241 (13) mU/l during the infusion of 100 mU/m2/min.
HAEMODYNAMIC MEASUREMENTS
Heart rate and blood pressure
Figure 1 shows the changes in heart rate on the control day and the study day. There was no difference in baseline heart rate on the control day, 62 (3) beats/min, and on the study day, 64 (4) beats/min. In response to the low dose insulin infusion heart rate fell by a maximum of 4.6 (1.4) beats/min (p < 0.05v placebo) and by a maximum of 5.1 (1.3) beats/min during the high dose infusion (p < 0.005v placebo). Mean arterial pressure fell in response to insulin by 5.5 (1.5) mm Hg (p < 0.005v placebo) during the low dose infusion and 6.9 (2.2) mm Hg (p < 0.01 v placebo) during the high dose infusion.
Change in heart rate (ANOVA p = 0.004). In this and figs 2-6, Insulin 40 and Insulin 100 refer to measurements taken during insulin infusions of 40 and 100 mU/m2/min, respectively, after (A) 30 minutes and (B) 60 minutes. ANOVA refers to analysis of variance for repeated measures that was used to compare the effect of insulin doses with placebo. Symbols refer to post hoct tests comparing each dose of insulin infusion with placebo at each time point. *p < 0.05, **p < 0.01,***p < 0.005.
Cardiac output
Figure 2 shows the values for cardiac output. There was no difference in baseline cardiac output on the control, 2.9 (0.2) l/min, and study days, 2.8 (0.3) l/min. Insulin led to a modest increase in cardiac output by a maximum of 0.15 (0.09) l/min (p < 0.01v placebo) during the low dose infusion and 0.13 (0.11) l/min (p < 0.05 v placebo) during the high dose infusion.
Change in cardiac output (ANOVA p=0.025). For key to symbols see fig 1 caption.
Forearm blood flow
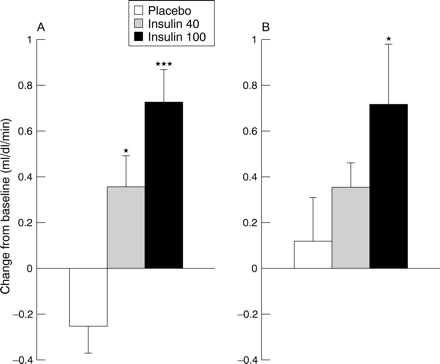
The changes in forearm blood flow are shown in fig 3. Baseline values were similar on the control day, 2.07 (0.18) ml/dl/min, and on the study day 2.37 (0.12) ml/dl/min. Insulin infusion had a highly significant effect on forearm blood flow compared with placebo (p < 0.001 for analysis of variance). Forearm blood flow increased by 0.36 (0.13) ml/dl/min after 30 minutes of the low dose infusion (p < 0.05 v placebo) and to 0.73 (0.14) ml/dl/min after 30 minutes of the high dose infusion (p < 0.005 v placebo).
Change in forearm blood flow (ANOVA p < 0.001). For key to symbols see fig 1 caption.
Figure 4 shows the changes in forearm vascular resistance. It decreased in response to both the low and the high dose infusions of insulin (p = 0.001 for analysis of variance).
Change in forearm vascular resistance (ANOVA p = 0.001). For key to symbols see fig 1 caption.
Superior mesenteric artery blood flow
Figures 5 and 6 show the changes in superior mesenteric artery haemodynamics. Superior mesenteric vascular resistance rose and superior mesenteric blood flow fell during the experiment. There was a non-significant trend towards a greater fall in splanchnic blood flow during the insulin infusion (p = 0.145 for analysis of variance).
Change in superior mesenteric flow (ANOVA p = NS). For key to symbols see fig 1 caption.
Change in superior mesenteric vascular resistance (ANOVA p = NS). For key to symbols see fig 1 caption.
PLASMA CATACHOLAMINES
Baseline plasma noradrenaline was 2.49 (0.09) nmol/l on the control day and 2.49 (0.08) nmol/l on the study day. There was no significant difference in the noradrenaline concentrations during the placebo and insulin infusions (p = 0.734 for analysis of variance).
Discussion
This study has shown that insulin has important and possibly unique central and peripheral haemodynamic effects in patients with chronic heart failure. It improved limb, and therefore skeletal muscle, blood flow by causing vasodilatation in the forearm vascular bed. There was a trend towards a reduction in blood flow to the gut that was probably secondary to the increased skeletal muscle blood flow and is a favourable redistribution of peripheral blood flow. Furthermore, a fall in heart rate and only a modest increase in cardiac output accompanied these changes. Insulin infusion had a negligible effect on sympathetic nervous system activity measured in terms of plasma noradrenaline concentrations.
Reduced blood flow to skeletal muscle has been shown to be a major determinant of exercise capability of patients with chronic heart failure.6 ,12 Agents such as ACE inhibitors and phosphodiesterase inhibitors increase blood flow to skeletal muscle and tend to improve exercise capability.7 ,13 ACE inhibitors have systemic vasodilatory properties without direct inotropic activity and have been shown to be of benefit in all grades of chronic heart failure.14 ,15 In contrast drugs such as the phosphodiesterase inhibitors improve muscle blood flow in part by increasing cardiac output by their inotropic activity and have consistently been shown to worsen survival.16 Therefore, if skeletal muscle blood flow can be increased by local vasodilator action without major direct effects on cardiac output or sympathetic nervous system activity, then there should be no detrimental effect on mortality. Furthermore, if this vasodilatation is selective for the skeletal muscle vascular bed and is associated with a reduction in and redirection of blood flow from less important vascular beds such as the gut this would be of added value. The changes we have described occur at doses of insulin chosen to produce physiological plasma concentrations—the low dose and supraphysiological concentrations. At physiological concentrations insulin causes selective skeletal muscle vasodilatation and is associated with advantageous redistribution of peripheral blood flow. It appears to be unique in this respect.
The reduction in heart rate is interesting. In normal volunteers heart rate tends to increase with insulin probably because of increased sympathetic nervous activity.17 In our experiment insulin had no effect on plasma noradrenaline concentrations compared with placebo. The beneficial reduction in heart rate we observed possibly occurs because patients already have a high sympathetic tone and the vasodilator effects of insulin reduce this sympathetic drive. However, the relatively low mean resting heart rate, in the absence of β blockade, in this cohort suggests that sympathetic activation at baseline was not particularly notable. An alternative explanation for the fall in heart rate would be a hitherto unrecognised effect of insulin on the parasympathetic system. Plasma noradrenaline concentration is an insensitive measure of changes in sympathetic activity. Further studies of autonomic activity by means of more robust methods such as microneurography in the face of insulin infusion in a similar cohort of patients would be of considerable interest.
Baseline measurements suggest that the study group had significantly impaired left ventricular function with reduced ejection fraction and low cardiac output. The mean resting cardiac output of approximately 3 l/min is lower than might be expected in patients with only moderate symptoms of heart failure and may be an underestimate. The study group was, however, older than most groups with heart failure in previous studies using invasive haemodynamic measurements and this may be a contributing factor to the low resting cardiac output. Our method of non-invasive measurement of cardiac output has been well validated and widely used, and is, we believe, robust. Since there was a modest increase in cardiac output in the face of a fall in heart rate, by extrapolation there must have been an increase in stroke volume during the insulin infusion. This may result from altered loading conditions on the heart caused by a reduction in systemic vascular resistance but this hypothesis must be confirmed by further, probably invasive, studies of central haemodynamics.
Insulin resistance has been identified as a component of the neurohormonal disturbance associated with chronic heart failure.1 ,2 Resistance to the metabolic action of insulin leads to a state of chronic overproduction of endogenous insulin to maintain normal blood glucose concentrations. It has been suggested that this state of hyperinsulinaemia may have beneficial haemodynamic effects in patients with chronic heart failure. This has been supported by a correlation between insulin resistance and skeletal muscle blood flow. Our results support this finding by confirming the integrity of insulin's vascular action in patients with chronic heart failure. However, little is known about the effect of chronic hyperinsulinaemia on muscle function during exercise and this remains an area for further study.
Regardless of the importance of endogenous overproduction of insulin our results raise fascinating questions about the use of exogenous insulin infusion in the management of patients with heart failure.
Insulin infusions have been used in the past to treat severe heart failure although this approach has never been subjected to a controlled trial and the mechanism of action has never been investigated in detail.18 A beneficial role for insulin infusion in patients with severe left ventricular dysfunction following cardiopulmonary bypass has also been proposed.19Glucose-insulin-potassium infusion following acute myocardial infarction was advocated in the 1960s but has never gained widespread acceptance. Interest in this treatment has been rekindled by a favourable meta-analysis and by the randomised, controlled DIGAMI (diabetes mellitus insulin-glucose infusion in acute myocardial infarction) trial, which elegantly showed the beneficial effect of early insulin treatment in diabetic patients with acute myocardial infarction.20 ,21 Discussion of these studies has largely centred on the metabolic effects of glucose, insulin, and potassium infusions.
The beneficial effects of insulin in these settings have been assumed to come from a protective effect on myocardial metabolism. We have shown that in patients with chronic heart failure insulin is a selective peripheral vasodilator with a modest effect on cardiac output. Insulin has no effect on sympathetic nervous system activity and leads to a reduction in heart rate. Our results show that insulin has significant haemodynamics effects that may conceivably influence the outcome of patients with heart failure or acute myocardial infarction. Further studies focusing on the haemodynamic actions of insulin in the setting of severe decompensated heart failure, acute myocardial infarction, and cardiac surgery would be of great interest.
Acknowledgments
This study was funded by a grant from the Special Trustees of University Hospital, Nottingham, UK.