Article Text
Abstract
Objective To determine the prevalence and spectrum of congenital heart disease (CHD) and the impact of a national prenatal ultrasound screening programme on outcome in a well-characterised population.
Design and setting A comprehensive registry was created of all paediatric and fetal patients with CHD over a 21-year period (1986–2006) in the Czech Republic. The centralised healthcare system enabled confirmation of prenatal and postnatal findings clinically and by post mortem.
Patients and results In the entire cohort of 9475 fetuses referred for detailed cardiac evaluation, 1604 (16.9%) had CHD, of which 501 (31.2%) had additional extracardiac anomalies. In the pregnancies which continued, 59 (8.6%) of 685 fetuses died in utero, and 626 (91.4%) babies were born alive. Prenatal detection rate was highest in double outlet right ventricle (77.3%) and hypoplastic left heart (50.6%). Detection rate increased significantly (p<0.001) for 12/17 lesions comparing 1986–1999 and 2000–2006. In recent years, detection of hypoplastic left heart reached 95.8% while transposition of the great arteries was diagnosed antenatally in only 25.6%.
Conclusion The nationwide prenatal ultrasound screening programme enabled detection of major cardiac abnormalities in 1/3 of patients born with any CHD and 80% of those with critical forms. Nevertheless, owing to the severity of lesions and associated extracardiac anomalies, the overall mortality of antenatally diagnosed CHD remains high. These findings are important for the understanding natural history of CHD for the establishing of screening programmes in Europe.
- Fetal echocardiography
- congenital heart disease
- ultrasound screening
- epidemiology
- paediatric cardiology
- echocardiography-fetal
- epidemiology
Statistics from Altmetric.com
- Fetal echocardiography
- congenital heart disease
- ultrasound screening
- epidemiology
- paediatric cardiology
- echocardiography-fetal
- epidemiology
Introduction
Antenatal detection of congenital heart disease (CHD) by cross-sectional echocardiography has been possible for almost 20 years and has provided new information on the early evolution of cardiac malformations. However, all studies have reported on highly selected cohorts, with varied, uncertain inclusion criteria and are thus not representative of the prevalence of malformations and their outcome. Since 1986, in the Czech Republic, the centralised healthcare system enabled the creation of a comprehensive national registry of all prenatal and paediatric patients with CHD.1–3 Furthermore, all pregnant women during this time underwent fetal ultrasound scanning by obstetricians who had previously undertaken a structured training programme provided by paediatric cardiologists.4–6 All prenatal and postnatal findings could be confirmed, as all the patients were managed in a single tertiary referral centre and a post mortem was obligatory in any child who died either at home or in hospital (including pregnancies which were terminated).
This significant investment in organisation of early diagnosis and care has enabled us to report, for the first time, the prevalence and spectrum of CHD and the impact of a nationwide screening programme on outcome in a large, representative, unselected population. We are also able to compare the antenatal prevalence with that previously established in the population for detection rate of individual conditions and the impact of antenatal screening on their outcomes. These findings show a different spectrum, prevalence and outcome of the heart lesions and are crucial for planning the future of CHD services in Europe.
Methods
Postnatal prevalence of CHD and prevalence study protocol
The region of the study was Bohemia (52 478 km2 in size, with an ethnically uniform population of 6.314 million with negligible migration), which represents two-thirds of the Czech Republic. All 815 569 children born alive 1980 and 1990 entered the study.1 Paediatricians examined all children immediately after birth, at the end of the first postnatal week and at least four times during infancy as part of national screening which ended at 3 years of age. All children suspected of having any cardiac abnormality were referred to a paediatric cardiologist.
The care of children with CHD in Bohemia is centralised in one specialised centre providing final detailed diagnosis, treatment (including surgery and catheter interventions) and long-term follow-up of all paediatric patients with heart disease. A paediatrician with an interest in paediatric cardiology works in each district and the network of these district specialists is headed by a provincial department of paediatric hospitals situated at a teaching hospital. A detailed clinical examination, ECG, echocardiography, CT and MRI and/or cardiac catheterisation with angiography proved or disproved heart disease in all cases with suspected CHD. There was a legal requirement for all children who died either at home or in a hospital (including those with unrecognised heart lesions) to undergo a postmortem examination.7 8 Isolated dextrocardia, non-stenotic bicuspid aortic or pulmonary valve and asymptomatic vascular abnormalities were not included. CHD causing congestive heart failure, severe cyanosis, acute life-threatening lesions and autopsy-proven CHD causing death in infancy (<12 months of age) were classified as ‘critical’.9
Prenatal CHD prevalence study protocol and organisation of fetal screening
Nationwide prenatal screening with echocardiography was performed between 18 and 21 weeks of gestation (government-guaranteed second-level prenatal ultrasound scan programme) by a local obstetrician/gynaecologist in every resident pregnant woman within the Czech Republic. A routine ultrasound scan was based on two-dimensional imaging to obtain a good quality four-chamber view and to visualise the crossing of both the great arteries according to published guidelines.4–6
Obstetricians interested in prenatal cardiac scanning were trained by specialised prenatal cardiologists and further close cooperation with district paediatric cardiologists specialised in fetal echocardiography was established. This enabled continuing education of every gynaecologist performing prenatal cardiac scanning. The district prenatal cardiologists provided further screening of suspected cases with the elimination of most false-positive findings. Fetuses suspected as having CHD were referred to the centre for final evaluation, which was performed exclusively by experienced paediatric cardiologists. Echocardiographic examination was completed by subsequent gynaecological ultrasound investigation, either to exclude or to define any extracardiac abnormality. Genetic examination, including karyotyping, was offered and complete counselling was carried out. All fetuses born alive with significant CHD were admitted to the intensive care unit immediately after delivery. The prenatal diagnosis was confirmed by clinical examination and detailed echocardiographic investigation. Further information was obtained by cardiac catheterisation and angiography if needed, and by intraoperative findings on all patients who underwent surgery.
A postmortem examination was performed by a fetal pathologist experienced in cardiovascular malformations on all fetuses terminated before 24 weeks’ gestation or who died antenatally or postnatally. This legal requirement enabled comparison with the diagnosis made during cardiac screening.
Classification of CHD
Complex cardiac abnormalities, including atrial isomerism, were classified according to the dominant heart lesion. In all complex heart lesions, atrioventricular septal defect was a primary diagnostic lesion. Double outlet right ventricle was diagnosed if the aorta or pulmonary trunk over-rode the ventricular septal defect by >50% on echocardiogram, post mortem or any additional imaging modality. We classified a ‘single ventricle’ as univentricular atrioventricular connection with double inlet or common atrioventricular valves. When an atrioventricular connection was absent, the diagnosis of either tricuspid or mitral atresia was established. ‘Hypoplastic left heart syndrome’ was defined as a heart with small left ventricle and flow reversal in the aortic arch (with or without a ventricular septal defect).
Assessment of fetal heart screening effectiveness
To assess the effectiveness and impact of fetal cardiac screening, the number of fetuses with antenatally diagnosed heart lesions was compared with the estimated number of children who would have been born with the same heart condition, based on the prevalence of those lesions within the Czech Republic between 1986 and 2006. Postnatal prevalence of individual heart lesions was calculated from the data in the nationwide surveys published by Šamánek et al1 2 7 8 The demographic data were obtained from the national database (the Institution of Health Information System) affiliated to the Ministry of Health Care of the Czech Republic. Antenatal diagnosis was confirmed or modified by a postmortem study in all fetuses undergoing early termination and in those who died ‘in utero’ or postnatally, whether treated or not. All children born alive with an abnormal antenatal scan were examined by experienced neonatologists and paediatricians. Paediatric cardiologists assessed those children presenting with a murmur or any other cardiac symptoms. All children with any CHD requiring expert diagnosis and all those referred for intervention (surgery, catheter) returned to the specialist centre and examination of prenatal findings was a routine part of the patient's evaluation (figure 1).
Floating chart of diagnostic assessment following fetal diagnosis of congenital heart disease.
Statistical assessment
Statistical analysis was performed using SigmaStat 3.5, Systat Software Inc (San Jose, California, USA). Data were expressed as frequencies or mean and SD. Paired t test and the Mann–Whitney sum rank test were used to compare pre- and postnatal frequency of CHD, and Fisher exact test was used to compare prenatal detection rates between two time periods. Probability values of p<0.05 were considered significant.
Results
Prevalence and spectrum of prenatally diagnosed CHD
In 8875 consecutive pregnant women, 10 948 prenatal echocardiographic scans were performed in 9475 fetuses of age ranging from 14 to 41 weeks of gestation (median 23 weeks of gestation). In the entire cohort of 9475 scanned fetuses, 1604 had confirmed CHD (16.9%); additional extracardiac anomalies were found in 501 fetuses (31.2%). Most common lesions were atrioventricular septal defect occurring in 243 cases (15.1%), hypoplastic left heart syndrome in 241 (15.0%), ventricular septal defect in 146 (9.1%) and double outlet right ventricle in 140 (8.7%) fetuses (table 1). Pulmonary atresia was diagnosed in 97 cases (6.0%), transposition of the great arteries in 87 cases (5.4%) and tetralogy of Fallot in 83 (5.2%). Neither isolated total pulmonary venous connection nor anomalous origin of left coronary artery from pulmonary trunk were diagnosed prenatally.
Spectrum of prenatally diagnosed congenital heart diseases (abbreviations of individual lesions)
Prenatal frequency of individual heart anomalies differed significantly from the postnatal frequency (figure 2). Postnatally, the most common cardiac lesion, ventricular septal defect (41.6% of all CHD), was detected in only 9.1% of all prenatally examined heart lesions, aortic stenosis (postnatal frequency 7.8%) was diagnosed prenatally in 4.9%. The proportion of postnatal and prenatal frequency of transposition of the great arteries was equal (5.4%). For the hypoplastic left heart, atrioventricular septal defect and double outlet right ventricle the prenatal frequency was higher (15.0%, 15.1%, 8.7%, respectively) than the postnatal frequency (3.9%, 3.9%, and 1.1%, respectively).
Frequency of individual cardiac lesions diagnosed prenatally compared with postnatal frequency. AS, aortic stenosis; AVSD, atrioventricular septal defect; COA, coarctation of aorta; DORV, double outlet right ventricle; EBST, Ebstein anomaly; HLH, hypoplastic left heart; PA, pulmonary atresia; PS, pulmonary stenosis; PTA, persistent arterial trunk; SV, single ventricle; TA, tricuspid atresia; TGA, transposition of great arteries; TOF, tetralogy of Fallot; VSD, ventricular septal defect.
Fetal heart screening effectiveness
Effectiveness of fetal screening over 21-years of follow-up
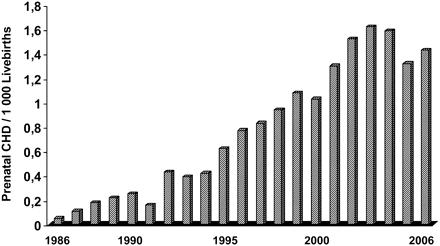
Between 1986 and 2006, 2 269 715 children were born alive in the Czech Republic. Of these, 15 162 children were estimated to be born with CHD (6.68/1000 live births). The 1906 subjects, estimated to be born with an atrial septal defect (prevalence 0.53/1000 live births), and those with patent arterial duct (prevalence 0.31/1000 live births) were excluded from this part of the study protocol. Of the remaining 13 256 children assumed to be born with CHD, 4653 children were expected to be born with a ‘critical’ form of CHD (35.1% of all CHD, 2.3/1000 live births). Between 1986 and 2006, 1604 fetuses were diagnosed prenatally giving an overall prenatal detection rate of 12.1%, and 34.5% of estimated critical lesions. This prenatal detection rate increased over the years from only 0.6% overall and 2.3% of ‘critical’ CHD in 1986 to 28.5% overall and 80.7% of ‘critical’ CHD in 2003 (figure 3). The estimated prevalence of antenatally diagnosed CHD increased from 0.05 per 1000 children born alive in 1986 to 1.62 in 2003 and 1.43 cardiac abnormalities per 1000 live births in 2006 (figure 4).
Prenatal detection of congenital heart disease (CHD) compared with calculated numbers of children expected to be born with any CHD, and with critical forms of cardiac abnormalities over 21-year follow-up.
Calculated prenatal prevalence of congenital heart disease (CHD) per 1000 children born alive between 1986 and 2006.
Prenatal detection of individual heart lesions
The prenatal detection rate was examined for a range of individual lesions, including atrial septal defect (table 2). Prenatal detection rate in the total cohort over 21 years was high in double outlet right ventricle (77.3%), hypoplastic left heart (50.6%), Ebstein`s anomaly (50.0%), atrioventricular septal defect (42.9%) and single ventricle (42.5%). In contrast, none of the fetuses with the isolated form of total anomalous pulmonary venous drainage and anomalous origin of left coronary artery from pulmonary trunk were diagnosed prenatally, and prenatal detection rate was low in atrial (0.8%) and ventricular (2.4%) septal defects and in pulmonary stenosis (3.2%). There was marked improvement in the detection rate for 12/17 individual heart abnormalities comparing data before 1999 with the data from later period. Between 1986 and 1999, hypoplastic left heart was diagnosed prenatally in 31.4% compared with 95.8% of fetuses diagnosed between 2000 and 2006 (p<0.001), transposition of the great arteries was antenatally revealed in only 5.7% between 1986 and 1999 in contrast to 25.6% (p<0.001) of fetuses diagnosed between 2000 and 2006. Significantly higher detection rate after 1999 was achieved also in tricuspid atresia (90.9%), atrioventricular septal defect (83.4%), persistent arterial trunk (80.8%), Ebstein`s anomaly (70.0%), interrupted aortic arch (69.2%), pulmonary atresia (68.2%), tetralogy of Fallot (37.3%), coarctation of aorta (19.7%), aortic stenosis (14.5%), pulmonary stenosis (7.4%) and ventricular septal defect (5.1%). Seventy-two fetuses with double outlet right ventricle were diagnosed prenatally between 2000 and 2006, however only 54 children were estimated to be born alive with the same lesion.
Prenatal detection rate of individual congenital heart diseases over 21 years of follow-up and comparing two time periods, 1986–1999 and 2000–2006
Outcome of prenatally diagnosed heart lesions
Families opted for early termination in 919 (57.3%) of the 1604 fetuses with CHD (figure 5). Of 685 fetuses for which pregnancy was continued, 59 (8.6%, 3.7% of all) fetuses died before reaching term and 626 babies were born alive (91.4% of continuing pregnancy, 39% of antenatally detected cardiac abnormalities). Despite optimal delivery, management and expert treatment, another 147 children died later. As a result, only 479 (29.9%) of the 1604 prenatally affected fetuses were alive at the end of the study (table 3). This survival rate increased over the 21 years of the study period: in the first 11 years (1986–1996), survival rate was as low as 42.2% (54/128 children born alive), and rose to 85.3% (425/498 children) over the following decade (1997–2006).
Outcome of pregnancies with prenatally diagnosed congenital heart disease (associated extra cardiac anomalies in 501 fetuses (31.2%)).
Outcome of pregnancies with prenatally diagnosed congenital heart disease over twenty-one year follow-up
Discussion
The centralised healthcare system operating in the Czech Republic has provided comprehensive data on the prenatal prevalence of CHD and its impact on postnatal outcome. In this study, we have been able to describe the detection rates for CHD as closely as is feasible, because of a number of unique factors: a meticulous nationwide screening programme which enabled the creation of nation prenatal registry, availability of postnatal data facilitated by thorough screening of the entire paediatric population for CHD, single-centre tertiary referral and obligatory post mortem. The creation of a registry of congenital cardiac abnormalities that includes lesions detected both pre- and postnatally has provided, for the first time, an opportunity to assess the prevalence of CHD and its impact on postnatal outcome.
The prenatal and postnatal spectrum of CHD differed significantly and there was large variation in detection rates of individual lesions. One explanation is the natural history of different lesions during gestation with some developing and progressing, and others leading to death in utero. Technical-methodological factors are likely to influence detection, including the reduced resolution of fetal ultrasound equipment, resulting in the future detection of minor lesions, and the higher level of operator expertise required for fetal scanning.
It is well known that the prevalence of cardiac abnormalities is higher in early gestation and these lesions are an important cause of very early miscarriages.10–12 In our series, the intrauterine death rate of continuing pregnancies with CHD detected in mid-trimester was 8.6%, and this is similar to that reported previously.13–16 Among fetuses that died from isolated CHD we found a range of cardiac abnormalities that is rarely seen postnatally. The rare cases of congenital absence of the aortic valve,17 double outlet right ventricle with intact ventricular septum and severe mitral regurgitation and unguarded tricuspid valve are among the intrauterine deaths in our series. These are either not seen or are extremely rare postnatally as the fetuses almost always die from severe fetoplacental failure at an early gestational age.
The prevalence and antenatal detection of cardiac lesions has differed in previous reports. In our series, hypoplastic left heart was detected in 50% of expected cases compared with 16% in a UK population,18 28% in the USA16 and 30% in France.19 However, in recent years, prenatal detection of some of the heart lesions has been very high: 95.8% in this study, and 88.9% in a study from Paris.20 We had a relatively low rate of prenatal detection of aortic coarctation (7.8%) compared with the British study,13 and this remains challenging if not impossible in some cases.21 22 For most lesions, detection rates have improved markedly with time. Residual differences in the reports (eg, our 26% detection of transposition versus 72% in a Paris region20) are likely to be due to the level of training of the echocardiographers.
The change in expertise, equipment and training over two decades was a contributing factor to the overall improvement in detection rate of CHD; however, neither of these dramatically improved detection rate of several abnormalities. Our data suggest that detection rates for some lesions such as transposition of the great arteries, total anomalous pulmonary venous drainage, ventricular septal defects and mild aortic and pulmonary stenosis could be improved by involving paediatric cardiologists specialised in fetal cardiac scanning in the screening programme. This is not likely, however, to be the best approach and a widely acceptable high-level programme to create a group of specialist cardiac sonographers may be a more realistic strategy in most countries.
Despite the clear advantages of a national comprehensive approach, identification of all cases of CHD and thus determination of the true ‘incidence’ remains difficult. We could not re-examine all 8497 survivors from all the 9745 subjects seen prenatally. It is very likely that some clinically insignificant minor lesions may have been dealt with by local paediatricians who were not included in the central registry of the CHD network during the study. Furthermore, despite the autopsy regulations, it was not possible to examine all fetuses that died at early stages either naturally or as a result of termination.
Despite the immense investment in this national prenatal cardiac screening programme, the survival rate for the affected fetuses remained low with only one-third alive at the end of the study period. The high rate of associated extracardiac anomalies has a significant impact on the outcome of affected pregnancies. In our entire cohort of 1604 of prenatally confirmed CHD, 31.2% had additional lesions (structural and chromosomal). Similar observations with significant reduction of survival rate have been found in Italian23 and British studies.24 25 Another multicentre Italian study,15 documented a 68% mortality rate in fetuses with heart lesions and additional non-cardiac structural abnormities, and a 74% mortality in those with additional chromosomal aberrations. In our series, the highest prenatal detection rate was in the double outlet right ventricle: between 2000 and 2006, prenatal prevalence was even higher than after birth, and there was a very high proportion of associated extracardiac anomalies (60.2%).26 27 We assume that a high proportion of fetuses with double outlet right ventricle and aneuploidy (most frequently trisomy 18 in our series) did not reach term. We are unable to prove this, however, owing to a high number of early terminations in our series. Our termination rate of 57% is relatively high compared with other large studies,13 14 20 23 26 and, in contrast to them, there has been no significant decline over the past two decades.
Although termination of pregnancy has never been offered at the time of counselling, the family's decision whether or not to continue was influenced by multiple factors, including religion and socioeconomic background. Nevertheless, we believe that compassionate care (including early termination) should be considered in the most unfavourable cases of CHD such a hypoplastic left heart or common arterial trunk with severe truncal regurgitation and those with CHD associated with a major or complex extracardiac lesion.
While our data show that antenatal diagnosis has had little impact on surgical outcome, it may significantly improve morbidity.28–33 Transport in utero, with the child delivered at a specialised tertiary institution may ensure a better clinical state of the neonate before cardiac intervention. Neurological outcome of babies with critical CHD diagnosed antenatally is better than for those without antenatal diagnosis29 31 despite of no difference in early surgical mortality. It has been more than 17 years since the world's first prenatal cardiac intervention was performed in the United Kingdom.34 Fetal cardiac intervention had appeared to be a promising form of treatment, based on the assumption that early fetal intervention might have a positive impact on the natural history of the disease and, thus, postnatal outcome. The results of intrauterine transcatheter balloon angioplasty in critical aortic and pulmonary stenosis, however, have been disappointing.35–37 Extensive determination of the risk to the mother and the fetus versus potential long-term benefits for the patient in an era of improving postnatal Norwood operation requires a large cohort of patients, technical skills and experience, and also precise long-term postnatal ascertainment. We strongly believe, that the centralisation of fetal interventions into a limited number of well-equipped centres (as documented by the Boston experience38 39) may help to better understand the natural history of obstructive cardiac lesions and to improve the results by optimising risk management assessment, patient selection criteria and technique revalidation.
These data have not been evaluated for the cost effectiveness that would accompany comprehensive fetal screening and we cannot recommend implementation of similar national screening programmes for financial reasons. Costs will vary within nations because of salary and healthcare differences. The rate of termination of pregnancies is an important determinant of cost and this will also vary. Furthermore, cost-effectiveness analysis is defined as the cost to be sustained per unit of health outcomes achieved (typically years of life saved or quality-adjusted life-years). We cannot use this model for assessment of cost effectiveness of screening as we do not aim to implement it to detect the anomaly in order to prevent the affected fetus being born. The cost savings of delivering an infant in good condition without cardiovascular collapse and/or expensive emergency transport to a regional centre are difficult to calculate and to extrapolate to different countries.
Conclusion
The centralised healthcare system with comprehensive registry of all paediatric and prenatal patients with CHD, including those who died pre- or postnatally, has enabled us to provide detailed information on the prevalence and spectrum of CHD and the impact of a nationwide screening programme on outcome in a large representative unselected national population. These findings show a different disease spectrum in prevalence and outcome and are crucial for planning future CHD services in Europe. Antenatal detection of major congenital cardiac abnormalities may, in some countries, significantly reduce postnatal prevalence of complex CHD. Extreme investments in developing paediatric cardiac centres can than be transferred towards improving and optimising care of the exponentially increasing numbers of adults with CHD.
Study limitation
Correct assessment of the detection rate of prenatally screened fetuses remains, in large epidemiological studies, difficult if not impossible. We did not re-examine all 8497 survivors from all the 9745 subjects seen prenatally. Some clinically insignificant minor lesions may be have been dealt with by local paediatricians who w not entered on the central registry of CHD at the time of this study.
Acknowledgments
The authors are grateful to all regional paediatric cardiologists and obstetricians contributing to the programme.
References
Footnotes
Funding This work was supported by a grant from the Ministry of Health of the Czech Republic: MZO-0064203/6203.
Competing interests None.
Ethics approval This study was conducted with the approval of the University Hospital Motol, Prague, Czech Republic.
Provenance and peer review Not commissioned; externally peer reviewed.