Article Text
Statistics from Altmetric.com
Diastolic dysfunction has a major impact on symptom status, functional capacity, medical treatment, and prognosis in both systolic and diastolic heart failure (HF), irrespective of the cause.w1 w2 When systolic dysfunction is clearly present, the central clinical question concerns the presence or absence of elevated filling pressure; a restrictive filling pattern is highly specific for elevated pulmonary wedge pressure in this setting.1w3 The transmitral flow pattern is also predictive of outcome; non-reversibility of restrictive filling with treatment portends a very poor prognosis.2 Thus, diastolic evaluation is an important component of the evaluation of the patient with systolic left ventricular (LV) impairment.
IS ASSESSMENT OF DIASTOLIC FUNCTION NECESSARY?
Paradoxically, the role of diastolic function assessment is more difficult to define in patients with diastolic HF. Diastolic dysfunction is the predominant cardiac abnormality in this syndrome, which is associated with increased risk of hospitalisation and death.3 However, the American College of Cardiology/American Heart Association guidelines for the evaluation and management of HFw4 support a diagnosis of exclusion—that is, clinical evidence of HF with preserved systolic function. Indeed, such a definition of diastolic HF has been adopted by the majority of previous reports,w5 and is supported by the results of recent studies which indicate that the presence of diastolic dysfunction may be assumed in patients presenting with HF and normal LV ejection fraction (LVEF).4w6 w7 Zile and colleagues demonstrated that at least one abnormal index of diastolic function was present in patients with HF and normal systolic function. These data suggest that a diagnosis of diastolic HF may accurately be made as a diagnosis of exclusion,4 albeit in a highly selected population of relatively young, predominantly male patients who were scheduled to undergo cardiac catheterisation (contrasting with the large clinical population of elderly, hypertensive, predominantly female patients with HF and preserved systolic function). Nonetheless, a recent review has highlighted the disconnect between Doppler echo measurements and true diastolic properties of the left ventricle, and has questioned the prevailing assumption that HF with preserved systolic function is always caused by diastolic dysfunction.5
A third scenario is perhaps the most difficult. These patients present with exertional dyspnoea in the context of normal systolic function, and in this situation, symptoms may be ascribed to diastolic HF. The lack of specificity of HF symptoms has led to concern regarding the heterogeneity of patients who are labelled with diastolic HF based only upon the presence of clinical HF and normal systolic function; many such patients may in fact have non-HF causes of their clinical presentations.w8 For these reasons, recent guidelines have called for invasive determination of diastolic dysfunction to make a definite diagnosis of diastolic HF,6 although the feasibility of this approach is limited. Doppler echocardiography is ideally suited for assessment of diastolic function, being widely available, non-invasive, and less expensive than other techniques. Echocardiographic assessment of diastolic function makes the diagnosis of diastolic HF more specific, allows serial assessment of the response of diastolic dysfunction to treatment, and facilitates inclusion of more homogeneous populations into intervention trials. Echocardiography has a broader role by allowing exclusion of overt LV systolic dysfunction (LVEF < 50%), significant valvar dysfunction (such as mitral regurgitation or aortic stenosis), and pericardial disease. However, a significant limitation is the requirement for expert interpretation of multiple parameters that vary with loading conditions and often provide conflicting information. The clinical importance of the evaluation of diastolic function warrants a simplified approach to diastology that is broadly accessible to general cardiologists. This article will therefore attempt to clarify this complex area, focusing on the practical application of Doppler echocardiography for the clinical assessment of the dyspnoeic patient, both in patients with preserved and impaired systolic function.
DEFINING DIASTOLIC DYSFUNCTION WITH ECHOCARDIOGRAPHY
No comprehensive consensus regarding diagnostic echocardiographic criteria for diastolic HF has been reached, but guidelines have been proposed.7w9 The European Study Group on Diastolic HF has provided criteria which relate to abnormal LV relaxation, abnormal LV filling, or reduced LV diastolic distensibility based on transmitral and pulmonary vein Doppler data.7 However, even these guidelines do not necessarily provide for echocardiographic identification of specific subgroups of patients with pseudonormal or restrictive filling patterns, potentially reducing their sensitivity for the diagnosis of diastolic dysfunction in the absence of cardiac catheterisation data. They are further limited by failure to include newer echo techniques such as tissue Doppler imaging (TDI). The assessment of diastolic function should be based on a comprehensive echocardiographic study integrating all available two dimensional and Doppler data.8 Such an approach will be outlined below.
Assessment of transmitral flow
Echocardiographic evaluation of diastolic function has been traditionally performed by measurement of transmitral flow parameters including the early (E) and late (A) diastolic filling velocities, the E/A ratio, and the E deceleration time (DT) from an apical four chamber view with conventional pulsed wave Doppler (fig 1A).w9 The transmitral E wave is related to the time course of active LV relaxation which generates a pressure gradient from the left atrium through the LV inflow tract to the LV apex.w10 Early diastolic LV filling is therefore largely influenced by the interaction of left atrial compliance and the rate of ventricular relaxation. The peak E velocity may be increased by either elevated left atrial pressure (the cause of high E/A ratios in cardiac disease), or alternatively, by low LV minimal diastolic pressure caused by rapid LV relaxation (which drives the high E/A ratios typical of normal young adults).9
Pathophysiological characterisation of left ventricular (LV) filling patterns. (A) Normal transmitral flow in a patient in sinus rhythm. (B) Impaired relaxation with normal filling pressures. (C) Pseudonormal filling. (D) Restrictive filling.
Based upon age adjusted interpretation of the transmitral flow profile, diastolic function is initially classified as either normal, impaired relaxation, pseudonormal, restrictive (which may be reversible or non-reversible with preload reduction), or indeterminate (if normal or pseudonormal cannot be differentiated) (fig 1). These patterns of LV filling represent progressively worse diastolic dysfunction as the LV becomes increasingly abnormal. It is important to consider that increasing diastolic dysfunction is usually accompanied by a progressive increase in LV filling pressures, which in turn have a major impact on the transmitral flow profile. Slow or prolonged LV relaxation therefore causes a decrease in E velocity but at the same time contributes to elevation of left atrial pressure, which in turn tends to increase the E velocity.10 These opposing effects of left atrial pressure and LV relaxation are also operative on the E deceleration time, which tends to be prolonged by impaired LV relaxation and shortened by increased filling pressures. Thus the effects of diastolic dysfunction on the E/A ratio and E wave deceleration time become progressively compensated and then over-compensated by the effects of loading, resulting in a non-linear (in fact “U” shaped) relation between these indices and severity of diastolic dysfunction.11 The isovolumic relaxation time bears a similar relation to diastolic dysfunction and load and does not provide additional information. In individual patients therefore, the filling pattern can change from mild (impaired relaxation) to more severe (pseudonormal or restrictive) diastolic dysfunction with either a progression of the underlying pathophysiological process, or alteration of loading conditions (fig 2). Similarly, improvement in the Doppler filling profile may occur over a longer period with treatments targeting the underlying cause (fig 3). Thus transmitral flow parameters must be further interpreted in the light of LV loading. This requires either incorporation of alternative load dependent parameters, or use of newer less load dependent techniques (or preferably both). Our standard approach is to gather the pulmonary venous flow profile, the medial and lateral early diastolic mitral annular velocities by TDI (Ea), the E/Ea values, and the response of E/A to Valsalva manoeuvre.
Load dependence of the LV filling pattern. (A) Restrictive filling associated with increased preload in a patient with renal failure. (B) Following dialysis, removal of intravascular volume has reduced LV filling pressure and unmasked the underlying impaired relaxation pattern. The presence of “reversibility” of the restrictive filling pattern indicates less severe LV stiffening and is associated with a better prognosis.
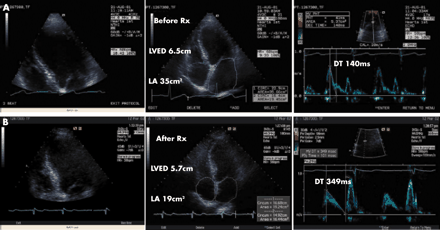
Improvement in the Doppler filling profile with therapy in dilated cardiomyopathy. (A) Ischaemic cardiomyopathy (LV ejection fraction 35%) with restrictive LV filling and New York Heart Association (NYHA) class III heart failure. (B) Improvement after seven months of medical treatment (LV ejection fraction 55%) with delayed relaxation pattern of LV filling consistent with normal filling pressures and NYHA class I–II. DT, E deceleration time; LA, left atrial area; LVED, left ventricular end diastolic dimension.
Tissue Doppler imaging: long axis relaxation rate
Long axis shortening (contraction) and lengthening (relaxation) of myocardial segments results in longitudinal motion of the mitral annulus toward or away from the (relatively fixed) LV apex during systole and diastole, respectively. Although long axis segmental shortening remains fairly uniform along the myocardial wall,w11 a gradient of increasing velocity from apex to base has been demonstrated.w12 w13 Mitral annular velocities may therefore be regarded as an “aggregate” of segmental myocardial velocities and in the absence of regional LV dysfunction accurately reflect global long axis LV function. The systolic velocity (Sa) corresponds to ventricular ejection while the early (Ea) and late (Aa) diastolic velocities correspond to the transmitral Doppler flow. In normal subjects the Ea occurs coincident with, or just before, the transmitral E wave, whereas in heart failure there is a progressive delay in Ea with respect to E.w10 Of more practical importance, the Ea velocity progressively decreases as the long axis relaxation rate becomes increasingly reduced in the setting of a wide range of cardiac disease processes including dilated, restrictive, and hypertrophic cardiomyopathies. Invasive studies have demonstrated that the Ea velocity correlates strongly with the time constant of isovolumic relaxation over a wide range of filling pressures.12–14 Specifically, the Ea velocity is much less susceptible to the effects of increased preload and remains low in patients with advanced diastolic dysfunction and pseudonormalisation of the transmitral E velocity.14w14 Further, Ea is typically lowest in patients with severe LV dysfunction and restrictive filling.
Pulsed wave TDI, whereby a sample volume (2–5 mm) is placed at the septal or lateral border of the mitral annulus in an apical four chamber view, is the most commonly used technique to record longitudinal velocities at the mitral annulus.11 Colour tissue Doppler is an alternative method, based on autocorrelation, which permits a colour velocity map to be gathered over the entire image sector, and therefore permits off-line measurement of annular or myocardial velocities at any point in the image. While pulsed wave Doppler offers superior temporal resolution, current colour Doppler algorithms can acquire data at high frame rate (> 100 frames per second) and generate clear velocity–time curves without the problem of gain dependent spectral broadening which affects the pulsed wave technique. However, because of its wider availability and large clinical evidence base, pulsed wave Doppler is the preferred technique for routine assessment of diastolic function in the clinical setting. Published normal ranges have been produced for both methods, with colour TDI velocities significantly lower compared with pulsed wave velocities.w15 w16 In keeping with the normal age dependent reduction in diastolic function, reference values for Ea must be adjusted for agew17 w18 (table 1).
Age adjusted normal cut offs for selected diastolic parameters.w9 w16 w27 w44 w96
A major advantage of TDI is its high feasibility, high reproducibility, and ease of application in the clinical setting.12,15 Velocities at both the septal and lateral mitral annulus may be obtained with minimal increase in the duration of the study. Recent evidence suggests that velocities at the septal and lateral annulus may be affected by different variables and are not interchangeable.w18 If obtaining only a single measurement, the lateral Ea may be preferredw19 as the septal Ea velocity has been demonstrated to be altered by preload in subjects with normal LV function,w20 although this effect may decrease as LV relaxation becomes progressively impaired.16 In addition, the septal Ea velocity may be influenced by right ventricular diastolic function. Potential pitfalls to be considered when acquiring and interpreting pulsed wave TDI signals include ensuring that the two dimensional image quality is optimised and that the ultrasound beam is well aligned (< 30°) with the direction of longitudinal motion (which may be more challenging at the lateral annulus). Finally, localised segmental hypokinesis in a given LV wall will result in reduced velocity of annular motion at the corresponding site, possibly leading to a spuriously low estimate of global LV function. In this situation it is recommended to obtain an average Ea from multiple annular sites.w19
Tissue Doppler imaging: estimation of LV filling pressures
Progressive diastolic dysfunction is associated with both impairment of LV relaxation and an increase in left atrial pressure. These concurrent events tend to have opposing effects on the transmitral E velocity, rendering it poorly predictive of either process. However, the E velocity (which increases with elevation of left atrial pressure) may be “corrected” for the degree of impairment in LV relaxation rate by relating it to the Ea velocity (which is a relatively load independent measure of reduced LV relaxation) to provide an index, the E/Ea ratio, which has been demonstrated to correlate with mean left atrial pressure.12 This concept has been validated in various clinical conditions including normal and impaired systolic function, tachycardia, atrial fibrillation, and hypertrophic cardiomyopathy.12,15w21–23 This application of TDI for estimation of LV filling pressures has significantly advanced the ability of the echocardiographer to distinguish normal from pseudonormal LV filling. It is particularly useful in difficult cases such as patients with early elevation of left atrial pressure whose filling profile is in a transition phase between impaired relaxation and pseudonormal filling (fig 4).
Transition phase between impaired relaxation and pseudonormal patterns caused by early increase in filling pressures. (A) Although the E/A ratio remains < 1, (B) elevation of filling pressure is evidenced by a high pulmonary venous A reversal velocity (44 cm/s), and (C) increased E/Ea ratio ( = 15). In this case the transmitral A wave is not shortened, probably because the E velocity is elevated at the start of atrial contraction.17
As discussed for assessment of LV relaxation rate, the lateral Ea may be preferable for E/Ea ratio estimation of filling pressure as more defined cut offs have been reported. Nagueh and colleagues demonstrated that E/Ea > 10 using the lateral mitral annular velocity reliably predicts a pulmonary capillary wedge pressure of > 12 mm Hg.12 In comparison, using the septal Ea velocity, Ommen and colleagues found that while pulmonary capillary wedge pressure is likely normal if the E/Ea ratio is < 8 and likely elevated if > 15, intermediate values were less useful.15 A recent report found lateral E/Ea to be superior to septal E/Ea for predicting wedge pressure when ejection fraction is > 50%,w19 although an average of both values is more accurate in the presence of regional dysfunction.w19 w24
Pulmonary venous flow
The pulmonary venous Doppler signal comprises “forward” systolic (S) and diastolic (D) velocities into the left atrium, and a “backwards” late diastolic A reversal wave corresponding to atrial contraction. The major factors influencing the pulmonary venous Doppler profile are illustrated in fig 5. The systolic flow wave is often biphasic; the “S1” occurs in response to atrial relaxation while the “S2” (often more dominant) is thought to be caused by mitral annular descent towards the ventricular apex resulting from long axis LV shortening, which increases left atrial capacity and reduces chamber pressure.w25 The pulmonary venous D flow occurs just after, and is largely determined by, the transmitral E wave.w26 In older adults, systolic flow is dominant such that S/D is > 1.w27 Like the E and A velocities in the transmitral Doppler profile, the pulmonary venous S/D ratio exhibits a non-linear relation to progressive diastolic dysfunction. Importantly, however, this load dependence can be used to advantage to aid correct interpretation of the transmitral flow pattern. Therefore in the early stages of diastolic dysfunction (evidenced by a low E/A ratio), the D velocity is low and S/D is > 1. As detailed in fig 5, the S velocity is also influenced by left atrial pressure. As such, increased left atrial pressure will tend to “blunt” the S flow and reduce the S/D flow ratio to < 1, particularly when filling pressures are notably raised.w28 w29 However, while a useful index in the setting of reduced LV ejection fraction, this criterion is relatively insensitive for the detection of elevated left atrial pressure when LV systolic function is preservedw30 as brisk mitral annular descent tends to maintain the systolic forward fraction of pulmonary venous flow. Preservation of atrial contractile function has a similar effect by fostering low left atrial preload at the onset of LV systole.
Determinants of the pulmonary venous Doppler profile. AF, atrial fibrillation; LAP, left atrial pressure; LVEDP, left ventricular end diastolic pressure.
The pulmonary venous A wave provides an additional tool for assessment of LV filling pressure and diastolic function. The peak A reversal velocity increases as resistance to atrial forward flow increases as a result of increased ventricular stiffness and/or end diastolic pressure, such that a peak velocity > 35 cm/s is suggestive of elevated filling pressures.w9 However, left atrial mechanical dysfunction often accompanies advanced diastolic dysfunction (particularly in association with paroxysmal atrial fibrillation) and may lead to low A reversal velocities. A more robust pulmonary venous parameter may be derived from the difference in the transmitral and pulmonary venous A wave durations. As LV compliance decreases and diastolic pressure rises, increased afterload on the left atrium tends to shorten the transmitral A wave, while its duration measured in the pulmonary vein may be increased. A difference in the respective durations of > 20–30 ms accurately predicts significant elevation of LV end diastolic pressurew28 and may be an early marker of transformation from impaired relaxation to a pseudonormal filling pattern (fig 6). The major advantage of this parameter is its utility in the setting of preserved LV systolic function,w30 w31 while the obvious limitation is the difficulty in acquisition of accurate measurements of the pulmonary venous A duration from a transthoracic window.15
Patient with previous anterior infarction demonstrating (A) transmitral flow, (B) pulmonary venous flow, and (C) mitral annular velocities. Note pronounced prolongation of the pulmonary venous A reversal duration relative to the transmitral A duration. In this case, elevation of filling pressures is supported by the E/Ea ratio (0.76/0.05).
Load altering manoeuvres
The principle behind this step is to remove the effects of preload compensation and thereby unmask the underlying relaxation abnormality. Thus the aim is to produce a transient lowering of left heart filling pressures which in the clinical setting is most practically achieved with the Valsalva manoeuvre, although a similar effect may be obtained with sublingual glyceryl trinitrate.w32 During the Valsalva manoeuvre, an initial minor increase in systemic blood pressure (caused by increased pulmonary venous return) is followed by a decrease in systemic venous return and a gradual decrease in stroke volume, leading, after a few cardiac cycles, to a reduction in left atrial and LV filling pressures and potential “conversion” of pseudonormal filling to an impaired relaxation pattern (fig 7).w33 This approach remains largely qualitative, and the required decrease in either the E velocity or the E/A ratio to reach a diagnostic threshold varies with different studies and will depend upon the baseline values for E and A, the quality of Valsalva, degree of patient effort, and other factors.15 Even in the research setting, ability to obtain adequate data may be particularly low,15w34 thus limiting the sensitivity of the technique. A reduction of E velocity by 50% or complete reversal of the E/A ratio to < 1w35 may be useful criteria, although other investigators have been unable to determine an accurate cutoff.w34 The Valsalva manoeuvre may also be less predictive of elevated LV filling pressure when ejection fraction is preserved.w31
Case study of pseudonormal filling demonstrating the utility of recording transmitral flow during the straining phase of Valsalva. In panel A1 transmitral flow demonstrates an impaired relaxation pattern, and continuous wave Doppler of the tricuspid regurgitant signal (A2) reveals mild pulmonary hypertension (right ventricular systolic pressure = 39 mm Hg + right atrial pressure). A repeat study performed 10 weeks later for progressive dyspnoea reveals a “normal” E/A pattern (B1) and significant pulmonary hypertension (B2) (right ventricular systolic pressure = 60 mm Hg + right atrial pressure). Suspected pseudonormal filling is confirmed by the Valsalva manoeuvre (B3): the underlying impaired relaxation pattern is unmasked by preload reduction, with reversal of the E/A ratio, prolongation of the E deceleration time, and lengthening of the A wave duration.
Flow propagation velocity
The high temporal and spatial resolution of colour Doppler M mode can be used to provide a two dimensional representation of the velocity of early diastolic filling as the bolus of blood propagates through the mitral valve towards the LV apex. This flow propagation velocity (Vp) can be measured from an apical four chamber view with the M mode beam aligned parallel to LV inflow.11 With colour Doppler activated and the aliasing velocity reduced, a sharp colour wavefront can be demarcated which represents progression of a bolus of blood towards the LV apex in response to early diastolic LV relaxation; Vp may be determined from the slope of this isovelocity line. Analogous to Ea, Vp correlates with invasive measurements of LV relaxation and has been shown to be relatively independent of loading conditions.w36 Similarly, the E/Vp ratio has been demonstrated to correlate with LV filling pressure.w37 More recently, however, Vp has been shown to be significantly influenced by LV systolic function, which may act to normalise Vp values in the presence of impaired LV relaxation.w16 w38 Other investigators have found Vp to be relatively more preload dependent in comparison with Ea in patients with normal systolic function.w39 Thus although the E/Vp ratio correlates well with pulmonary wedge pressure in the setting of LV dilatation and reduced ejection fraction, it may be limited in patients with normal systolic function and particularly those with small or hypertrophic ventricles—that is, the same conditions under which conventional Doppler data becomes less reliable.
We do not use this technique clinically because several practical technical issues contribute to significant inter-observer variability.w40 w41 The colour map of the early filling wave is often partially biphasic, with a near vertical initial column related to movement of blood already present in the LV as the mitral valve opens, followed by a second column which represents true LV inflow. Rather than the ideal straight line, the colour map of the aliased velocity is frequently curvilinear which makes accurate measurement problematic. Accuracy is also limited when the gradient is steep (high Vp) as minute adjustments in the slope of the line result in large changes in the value of Vp. Therefore, this technique is most useful when a satisfactory colour M mode signal is achievable in the setting of impaired LV ejection fraction.
CATEGORISATION OF DIASTOLIC DYSFUNCTION
Impaired relaxation
Recognition
Slowing and prolongation of LV relaxation becomes apparent at an early stage of LV dysfunction,w42 perhaps because this part of the cardiac cycle is metabolically very demanding. Impaired LV relaxation reduces the peak transmitral pressure gradient, thereby reducing the E velocity and E/A ratio (to < 1 in young patients, < 0.5 in the elderly).7 Continued slow or discoordinate LV relaxation maintains a low transmitral pressure gradient into mid diastole resulting in prolongation of the E deceleration slope (> 220 ms in young patients, > 280 ms in the elderly)7 (fig 1B). As left atrial pressure remains relatively normal at rest in this early stage of diastolic dysfunction, patients may have symptoms only with exertion, and transmitral flow may be close to normal at rest. Nonetheless, even this mild degree of diastolic dysfunction places patients at increased risk for adverse cardiovascular events.3w43 However, the functional significance of an impaired relaxation pattern of LV filling is less clear, and estimation of resting LV filling pressures in this group is independently predictive of exercise capacity.17
Effects of age and heart rate
As there is an age dependent decline in diastolic function in normal subjects,w9 w27 w44 all diastolic indices should be interpreted in conjunction with age. Sex differences may also be significant—for example, elderly females tend to have lower E/A ratios and longer E deceleration times compared with males.w9 In some ways, the distinction of “age related normal changes” from pathologic filling patterns seems artificial. Studies of backscatter and strain characteristics have shown abnormal tissue characterisation in association with abnormal filling, suggesting the involvement of an age related process such as fibrosis.w45
The patient’s heart rate may also influence the LV Doppler filling pattern. In elderly subjects in the Framingham heart study both the transmitral E velocity and E/A ratio were inversely related to heart rate,w46 suggesting that in mild disease, abnormal filling patterns follow the exhaustion of “filling reserve”. In contrast, exercise induced tachycardia resulted in proportional increases in both E and A (such that the E/A ratio remained unchanged) as well as an increase in the mitral annular Ea velocity in healthy younger subjects.w47 Importantly, Nagueh and colleagues have demonstrated that the lateral E/Ea ratio remains a relatively accurate measure of pulmonary wedge pressure in a mixed clinical population during sinus tachycardia.w21
Other causes of delayed relaxation
Regional LV dysfunction caused by ischaemia or abnormal activation may be associated with dyssynchronous relaxation, which has a major impact on the dynamics of early diastolic filling.18 Pacing induced asynchrony has been shown to decrease LV filling indices in an animal model,w48 while left bundle branch block is associated with prolongation of isovolumic relaxation and reduced filling time in patients with dilated cardiomyopathy.w49 Isolated left bundle branch block with normal global systolic function is also associated with alterations of LV filling parameters.w50 Therefore, cardiac pacing and left bundle branch block may preclude accurate assessment of delayed relaxation from the LV inflow pattern, as this technique relies on the assumption of regional uniformity. Asynchronous long axis LV relaxation is also common in patients with coronary artery disease and may in fact be the major cause of reduced E velocity and an abnormal relaxation pattern in these patients.w51 Finally, right ventricular overload and pulmonary hypertension can significantly influence LV filling patterns, possibly resulting from abnormal septal motion and changes in LV geometry.w52
Normal or pseudonormal filling
Recognition
The finding of apparently normal filling in the dyspnoeic patient may suggest one of two problems—either the filling pattern is pseudonormal, or the patient does not have heart failure. A normal transmitral flow pattern is age and sex dependent but may be generally characterised by an E/A ratio of 0.75–1.5 and a deceleration time of 160–260 ms. As discussed above, the entities of normal and pseudonormal filling cannot be distinguished on the basis of transmitral flow alone. The pseudonormal pattern occurs in advanced cardiac disease (often with concomitant systolic dysfunction) where progressive impairment of LV relaxation and compliance leads to elevation of LV filling pressures. As preload increases, the rise in left atrial pressure begins to increase (and therefore “pseudonormalise”) the E velocity while elevation of LV end diastolic pressure tends to favour earlier equilibration of the transmitral pressure gradient and therefore shortens the E deceleration time back into the normal range. In only one setting may the transmitral flow give the diagnosis—the typical pseudonormal filling pattern may become modified during slow heart rates, such that the underlying impaired LV relaxation may be discernible.w53 This may manifest as a distinct decrease in the gradient of the E wave deceleration slope (fig 8A), or alternatively, as a period of mid diastolic transmitral flow distinct from the E and A waves, corresponding to the underlying prolonged or dyssynchronous LV relaxation which is uncovered by the bradycardia (fig 8B).
Mid diastolic flow represents an atypical pattern of pseudonormal filling modified by bradycardia; two patterns (A) and (B) are seen. (A1) Transmitral flow at rest demonstrating E/A ratio > 1 with a distinct change in the slope of the E deceleration line. (A2) The E deceleration slope with Valsalva manoeuvre is similar to the slope of the mid diastolic flow deceleration in the resting Doppler; also note the short A wave duration which lengthens during Valsalva. (B1) A discrete period of diastolic forward flow after cessation of the E wave. (B2) The underlying impaired relaxation pattern is unmasked by preload reduction with the Valsalva manoeuvre. This pattern of mid diastolic flow is also discernable in fig 3A and 6.
Differentiating normal from pseudonormal LV filling
We take the following initial steps to distinguish normal from pseudonormal filling:
-
Integrate the clinical information—An E/A ratio of 2 with deceleration time of 160 ms is likely normal in a young adult with a structurally normal heart referred for palpitations, but is almost certainly pseudonormal in an elderly hypertensive patient who was referred for investigation of dyspnoea.
-
Consider the status of the left ventricle on two dimensional echo—Even if the E/A ratio and deceleration time are in the normal range, LV filling is unlikely to be normal if there is significant LV hypertrophy or LV dysfunction. In this setting, preserved E velocity is better explained by elevated left atrial pressure than by brisk relaxation and a rapid decline in LV diastolic pressure. This effect of LV “suction” on transmitral flow is important to consider when attempting to differentiate normal from pseudonormal filling in older patients with small LV cavities. When such individuals exhibit vigorous LV systolic function (for example, LVEF > 70%), a high E velocity and E/A ratio may reflect lower LV minimum diastolic pressure due to brisk elastic recoil of the LV rather than elevated left atrial pressure.w23 Such patients will also tend to have a high tissue velocity (since this is directly proportional to LV minimal pressure)w23 so that the E/Ea ratio (see below) will remain low, indicating that filling is in fact normal rather than pseudonormal.
-
Evaluate LA size—Left atrial size correlates with mean pulmonary wedge pressure19 and is therefore a relatively sensitive marker of chronic diastolic dysfunction.w54 Diastolic HF is not a plausible explanation for chronic dyspnoea if the left atrial size is normal. The disadvantage is, however, that specificity may be compromised by conditions such as atrial fibrillation and mitral valve disease which commonly cause left atrial dilatation.
The next steps in the discrimination of normal from pseudonormal LV filling involve acquisition of tissue Doppler, pulmonary venous flow, load altering manoeuvres, and (sometimes) measurement of inflow propagation.
Integration of (conflicting) echo Doppler parameters
The integration of the clinical and two dimensional echo information with a full complement of Doppler data usually allows reliable discrimination of pseudonormal from normal filling, as illustrated in fig 9. The most useful Doppler parameters for this purpose are listed in table 2. However, it should be emphasised that in contrast to their value with overt systolic dysfunction, several parameters of diastolic function have reduced accuracy when LV ejection fraction is preserved.w55 In this setting the E deceleration time correlates poorly with filling pressures, and the Valsalva manoeuvre, pulmonary venous S/D ratio, and flow propagation velocity are all relatively unreliable indicators of diastolic dysfunction. The most robust parameters for estimating filling pressure to aid interpretation of the transmitral Doppler profile when LV ejection fraction is normal are the E/Ea ratio and the difference in pulmonary venous and transmitral A wave durations.w31 Even so, diastolic function may remain inconclusive in occasional patients who present very contradictory data that cannot be resolved.
Useful criteria for differentiating normal from pseudonormal filling in adult subjects with normal left ventricular systolic function
Case study of a 67 year old hypertensive female with NYHA class II–III dyspnoea and left bundle branch block on ECG, revealing classical echo Doppler findings of pseudonormal filling. (A) Apical four chamber view demonstrating left atrial dilatation. Transmitral Doppler (B1) at rest shows a “normal” E/A pattern; (B2) during the strain phase of the Valsalva manoeuvre E velocity gradually decreases with no significant change in the A velocity; (B3) post-Valsalva there is reversion to the baseline pattern. (C) Pulmonary venous Doppler showing decreased systolic velocity relative to diastolic velocity, and atrial reversal velocity of 40 cm/s (both caused by elevation of filling pressures). (D) Tissue Doppler velocities at the mitral annulus showing reduced Ea velocity (5 cm/s) and increased E/Ea ratio ( = 16). S, D, and Arev, pulmonary venous systolic, diastolic, and atrial reversal velocities respectively.
Cardiac catheterisation
Invasive measurement of diastolic function is often invoked as the gold standard method for assessment of diastolic function.6 However the cost, complexity, and expertise required, as well as patient risk and lack of tolerability associated with such procedures, mean that cardiac catheterisation is rarely performed specifically to evaluate diastolic function. In addition, unlike echocardiography, cardiac catheterisation does not lend itself to serial assessment for monitoring disease progression and response to treatment.
Restrictive filling
Restrictive filling, characterised by a pronounced increase in the E/A ratio (> 2) and shortening of the E deceleration time (< 150 ms) is seen in the “sickest” ventricles and indicates severely reduced LV compliance and notable elevation of left atrial pressure. In the setting of preserved ejection fraction, restrictive filling usually indicates severe infiltrative myocardial disease such as cardiac amyloidosis rather than hypertensive heart disease. In clinical practice, restrictive filling is most commonly seen in association with LV dilatation and severe systolic dysfunction and is strongly predictive of mortality in this population, particularly if it is not reversible with treatment.2
Systolic dysfunction
When systolic dysfunction is clearly present, the central clinical question concerns the presence or absence of elevated filling pressure. Impaired LV relaxation without preload compensation indicates relatively normal filling pressures. In this situation, no further evaluation of diastology is necessary unless one is dealing with borderline abnormal values (for example LVEF 40%, E/A 0.8, deceleration time 250 ms), in which case one should proceed with more comprehensive assessment. However, if the E/A ratio and deceleration time appear relatively normal (or increased and shortened, respectively) despite pronounced systolic dysfunction, preserved E velocity likely reflects elevated left atrial pressure. Corroboration may be reliably obtained with TDI, as low mitral annular amplitude in the presence of systolic dysfunction results in reduced early diastolic lengthening rate,w51 and therefore a high E/Ea ratio in the presence of elevated left atrial pressure.
Effects of atrial fibrillation
As transmitral flow reflects the left atrial to LV pressure gradient, the pattern of ventricular filling may be notably influenced by both the compliance and contractile function of the atrium. This issue attains practical clinical significance in the setting of paroxysmal atrial fibrillation. The return of sinus rhythm is often accompanied by atrial stunning, a loss of mechanical function that is proportional to the duration of fibrillation as well as anatomical characteristics of the atrium,w56 and which may lead to inappropriate interpretation of diastolic function. Recent evidence indicates that Aa correlates well with quantitative methods of left atrial function.w57 Figure 10 illustrates abnormalities of diastolic echo Doppler parameters that are typically observed in the setting of impaired left atrial contractile function. In contrast, brisk left atrial function is evident in fig 8, resulting in a high transmitral A velocity, preserved systolic (S1) dominance, and high atrial reversal velocity in pulmonary venous flow, and a high Aa TDI velocity.
A 63 year old man with sick sinus syndrome and intermittent atrial fibrillation. The transmitral Doppler flow during normal respiration (A) is abnormal with an E/A ratio of > 2 and is suggestive of elevated filling pressures. The pulmonary venous Doppler profile (B) demonstrates blunting of the systolic forward velocity, further suggesting elevation of left atrial pressure. However, there was no E/A reversal with the Valsalva manoeuvre (C), and tissue Doppler imaging of the mitral annulus (DS) revealed a high Ea velocity (15 cm/s) and E/Ea ratio of < 5. This patient therefore has normal LV diastolic function and filling pressures, and the low transmitral A velocity, low pulmonary vein systolic velocity, and low Aa can all be explained by left atrial mechanical dysfunction.
Evaluation of diastolic function is also difficult when atrial fibrillation is sustained, although in patients with severe systolic dysfunction the E deceleration time correlates well with LV filling pressure.20w58 In particular, a very short deceleration time—for example, 120–140 ms—is strongly predictive of elevated pulmonary wedge pressure.w58 Combining transmitral and pulmonary vein Doppler data may also allow estimation of pulmonary wedge pressure in patients with systolic HF.w59 When LV systolic function is preserved in atrial fibrillation, estimation of filling pressure from transmitral flow remains possible, but requires more complicated Doppler indices.20 Of potentially more practical use, the TDI appears to retain diagnostic value in patients with atrial fibrillation. Sohn and colleagues demonstrated that Ea correlated with prolongation of tau and E/Ea predicted elevation of LV filling pressure, both at cut offs which are similar to those reported for patients in sinus rhythm.w22 Other investigators have confirmed a strong relation between Ea and tau and between E/Ea and LV end diastolic pressure in atrial fibrillation.
NEW DEVELOPMENTS IN DIASTOLIC FUNCTION ASSESSMENT
The use of resting data alone is an important limitation of the Doppler approach, especially early in the disease, when the heart is compensated at rest and symptoms occur only with activity. The evolving techniques that may help with this diagnosis include assessment of LV filling during exercise, B type natriuretic peptide (BNP), and tissue characterisation.
BNP for diagnosis of diastolic dysfunction
Given the complexity of the echocardiographic evaluation of LV diastolic function, non-invasive diagnosis would be greatly aided by a simpler approach. In particular, a blood test with acceptable accuracy would be a very valuable addition to the diagnostic armamentarium, and recent attention has turned to BNP for this purpose.
BNP is a peptide secreted from the ventricular myocardium in response to dilatation and increased intra-cavity pressure.w60 w61 Elevation of BNP has been associated with a range of cardiac abnormalities which can result in increased filling pressures.w62 In particular, elevation of BNP has been demonstrated in the setting of acute HF, and correlates with the degree of LV systolic dysfunction in this setting.w63 w64 A BNP of 100 pg/ml has been demonstrated to be accurate for the emergency room diagnosis of acute systolic heart failure.w64 In addition, there is increasing evidence that the N-terminal fragment of the pro-BNP molecule that is released during secretion of BNP from cardiac myocytes (so called N-terminal BNP) has similar diagnostic utility.
This relation of BNP to ventricular filling pressure in systolic HF implies that BNP might also have diagnostic potential in patients with diastolic HF, in whom symptoms are also related to elevated LV filling pressures. It also suggests that BNP might be more relevant for the diagnosis of clinical diastolic HF, which is usually associated with pseudonormal or restrictive filling patterns,w65 rather than for the detection of exertional dyspnoea attributed to diastolic dysfunction, which is often characterised by an impaired relaxation pattern and may not be associated with high filling pressures at rest.w34 Thus BNP is usually elevated in patients presenting to an emergency room with shortness of breath due to HF regardless of whether the ejection fraction is preserved or impaired and a normal BNP value has high negative predictive value in this setting.w66 Further, high BNP concentrations have been reported in HF patients with normal LVEF,w67 and in those with isolated LV diastolic dysfunction.w65 Even so, BNP is reportedly lower in HF patients with preserved ejection fraction compared to those who have systolic dysfunction.w66 This is relevant for the diagnosis of diastolic HF because population studies have demonstrated notable increases in BNP with age and female sex,w68 leading to significant overlap in BNP concentrations between dyspnoeic elderly patients with HF and preserved ejection fraction and similar patients without HF.w66 Therefore, a specific BNP cut off may not accurately discriminate diastolic HF from non-HF presentations in the elderly, particularly elderly women (in whom the condition is most prevalent).w69 In addition, since BNP has a short half life (approximately 20 minutes), the timing of sampling in relation to the patient’s symptoms may have a profound influence on the utility of BNP for the detection of diastolic HF. In particular, clinically stable or treated patients who are limited by exertional dyspnoea caused by mild diastolic dysfunction often have relatively normal resting LV filling pressures and may therefore have normal BNP concentrations at rest.w70 Therefore, given that reported mean BNP concentrations in diastolic HF have varied from 56 pg/ml in a community settingw67 to 413 pg/ml in acute hospital presentations,w66 it is difficult to apply a simple cut off. However, as a general guide, in symptomatic patients with preserved systolic function, diastolic HF may be considered unlikely if BNP is < 50 pg/ml, and likely if BNP is > 100 pg/ml.
Whether BNP has a wider role in the diagnosis of hypertensive heart disease remains unclear. While BNP has been reported to be increased in patients with hypertensionw71 and LV hypertrophy,w72–74 its ability to detect increased LV mass in a community setting was suboptimal.w75 Similarly, a recent study found that BNP was suboptimal for the identification of diastolic (or indeed systolic) dysfunction in more than 2000 subjects randomly selected from the population.21
Responses of LV filling and BNP to stress
Since chronic stable diastolic HF is characterised by abnormal increases in diastolic pressures during exertion, exercise criteria would seem to be an important part of the diagnostic criteria. Indeed, if a patient demonstrates a high functional capacity during exercise testing then a diagnosis of chronic HF of any cause can be reliably excluded. An ideal test for the diagnosis of diastolic HF would allow objective demonstration of reduced exercise capacity caused by limiting dyspnoea while simultaneously confirming elevation of left atrial pressure. While cardiac catheterisation during exercise is not practical, non-invasive estimation of filling pressures using the E/Ea ratio at rest and immediately after maximal exercise is a potentially useful approach that has proved feasiblew47 w76 and warrants further investigation. Empirically, exercise limitation that is associated with conversion of an “impaired relaxation” pattern at rest to a “pseudonormal” pattern immediately post-exercise is indicative of elevation of left atrial pressure with exercise, and suggests that the slow LV relaxation is functionally important in a particular patient (fig 11). Similarly, augmentation of BNP with exercise might also have diagnostic potential in this situation. As a surrogate for LV filling pressures, increases in BNP during exercise may suggest elevation of LV filling pressures in a patient with exertional dyspnoea and suspected diastolic HF.w76
Change of LV filling pattern with exercise. The left panel suggests diastolic dysfunction with relatively normal filling pressures at rest. A change to a pseudonormal pattern associated with reduced exercise capacity suggests that the resting diastolic dysfunction is functionally significant in this particular patient.
Several other aspects of exercise echocardiography may provide clinically useful information. A hypertensive blood pressure response to stress is a preclinical marker of abnormal ventricular structure and function,w77 and contributes to exercise intolerance by inducing transient diastolic dysfunction.w78 w79 The presence of exercise induced regional hypokinesis implicates coronary artery disease as a cause of the patient’s symptoms, while increased pulmonary artery systolic pressure at peak exercise may be related to LV diastolic dysfunction.
Tissue characterisation
It is clear from the previous discussion that currently utilised techniques for the detection of hypertensive heart disease are relatively insensitive, demonstrating abnormalities only when extensive changes have occurred and when patients are already likely to be at considerably increased risk of adverse cardiovascular events. More sensitive echocardiographic techniques for characterising myocardial structure and function include strain imaging and assessment of integrated backscatter. Echocardiographic measurement of the extent (strain) and rate (strain rate) of segmental myocardial deformation utilises colour tissue Doppler to determine gradients between adjacent myocardial velocities.w80 While the clinical application of this recently developed technique is currently limited by a suboptimal signal to noise ratio and marked angle dependency, it has recently been applied in the research setting to identify reduced LV systolic function in patients with hypertension and isolated diastolic dysfunction.w81 In addition, its high sensitivity has potential to aid detection of preclinical myocardial dysfunction.w82 w83 Segmental myocardial properties may also be assessed by measurement of myocardial reflectivity with integrated backscatter. The magnitude of myocardial backscatter normalised to either the blood pool or pericardium, and the cyclic variation in backscatter with segmental contraction are related to both myocardial structure (degree of fibrosis)w84 and function.w85 Changes in these parameters precede functional changes in hypertensive LV hypertrophyw86 and also correlate with diastolic changes in the aging heart.w45 These sensitive techniques therefore provide an alternative means of identifying early end organ damage in hypertensive patients, and are currently being used to investigate diastolic function in various disease states.
In addition to providing specific information about the diastolic function of the ventricle, early diastolic mitral annular velocities have a potentially broader role in the detection of cardiac abnormalities. Conditions such as hypertension and diabetes contribute to global LV diastolic dysfunction, usually without significant regional heterogeneity. Thus mitral annular velocities, which represent the “sum” of long axis LV velocities, accurately reflect global LV function in these conditions. The resting Ea correlates with exercise capacity in hypertensive patients,w87 and has been demonstrated to be sensitive (more so than Sa) for the detection of myocardial dysfunction in various cardiac disease processes including hypertension,w88 diabetes,w89 myocardial infiltration,w90 and hypertrophic cardiomyopathy.w91 Moreover, Shan and colleagues have shown early diastolic myocardial velocity to be inversely related to interstitial fibrosis.w92 Finally, the TDI derived Ea velocity is independently related to cardiac mortality and provides predictive power which is incremental to data obtained from clinical and conventional Doppler assessment.22 The application of this approach is currently limited by deficiencies in understanding of the various factors which influence Ea,w18 and a lack of normal ranges with robust cut offs for identification of abnormal myocardial function.
Global versus segmental diastolic function
All of the techniques described above provide assessment of global LV diastolic function. However, it is clear from models of systolic dysfunction that it is possible to have normal global function (evidenced by preserved ejection fraction) in the presence of segmental hypokinesis, provided that enough normally contracting segments remain. This concept may also be applied to diastolic function, although at present it is unclear what extent (or severity) of segmental diastolic dysfunction is required before parameters of global diastolic function become abnormal. This regional non-uniformity has implications for the reporting of echo Doppler studies of LV diastolic function. If global systolic function (ejection fraction) is impaired then it can be assumed that global diastolic function is also abnormal and, for example, a “normal” E/A ratio would likely represent pseudonormal filling. Alternatively, when regional hypokinesis is present in the setting of preserved ejection fraction—for example, localised inferior wall ischaemia—one can assume that the hypokinetic inferior segments will have abnormal relaxation and compliance; however, it is possible for global diastolic function to be either normal or abnormal in this setting, depending on the extent/severity of segmental diastolic dysfunction and the diastolic function of the non-infarcted segments. In addition, as discussed above, the extent of regional asynchrony in the timing of myocardial relaxation may significantly affect measures of global performance. Further work utilising regional information from strain imaging with both echocardiographyw93 and magnetic resonance taggingw94 will likely provide important insight into these questions.
Alternative imaging techniques
In symptomatic patients with limited echocardiographic windows or poor quality data (for example, inadequate pulmonary vein Doppler), alternative imaging techniques may provide useful information. Historically, radionuclide techniques have been used but these are limited by low frame rates, cycle length variability, and background lung blood pool attenuation. Magnetic resonance imaging has recently been demonstrated to be sensitive for the detection of global diastolic dysfunction in early diabetic heart disease.w95 However, while spatial resolution is excellent, diastolic function assessment with magnetic resonance may be limited by lower temporal resolution and further experience is required.
CONCLUSIONS
A comprehensive assessment of diastolic function and filling pressures should ideally include integration of all available two dimensional and Doppler data with relevant clinical information such as age, exercise capacity, and the presence of hypertension, coronary disease, or diabetes. As multiple parameters are used to assess diastolic function, each with imperfect sensitivity and specificity, discordant results in a given patient are relatively common. A busy echo lab should therefore favour acquisition of parameters which offer both accuracy and low inter-observer variability. At the very least, this requires assessment of transmitral Doppler flow (which remains central to categorisation of diastolic function) in combination with newer, less load dependent Doppler echo techniques. Of available methods, pulsed wave TDI is the easiest to use and provides robust, relatively unambiguous, well validated data that are more reliable than the use of pulmonary vein Doppler, flow propagation velocity or load altering techniques, particularly when systolic function is preserved.15 In difficult cases, a comprehensive echo Doppler examination should be interpreted by an experienced echocardiographer and consideration should be given to further evaluation with BNP and exercise testing (fig 12). Thus, although echocardiographic evaluation of diastolic function in HF is complex, a simplified approach can be widely applied to provide important clinical and prognostic information for patients with and without overt LV systolic dysfunction.
Suggested schema for echo Doppler categorisation of diastolic function in patients with normal LV systolic function.
The clinical role of echocardiography for evaluating diastolic function is quite different depending on whether global LV systolic function (ejection fraction) is preserved or impaired. When the ejection fraction is greater than 45–50%, it is generally agreed that symptoms of HF should not be attributed to systolic dysfunction. In this setting, confirmation of diastolic dysfunction does not define diastolic HF, which still requires clinical correlation. Nonetheless, evidence of raised LA pressure supports a contribution from diastolic HF, and this has important prognostic and therapeutic implications. When global systolic dysfunction is present, accompanying diastolic dysfunction is assumed, and the principal aim of Doppler echocardiography is to estimate filling pressure, which again has direct implications for treatment and prognosis. A clinically useful classification for the reporting of diastolic function should therefore reflect these aims, and an example is given in table 3.
Practical classification of diastolic function
Deficiencies in understanding of the pathophysiology, epidemiology, and treatment of HF with preserved ejection fraction have been largely related to inability to accurately identify diastolic dysfunction with non-invasive techniques. Research over the past decade has seen a steady evolution of our understanding of diastolic function in a wide range of clinical scenarios. Parallel advances in ultrasound technology and its application in the non-invasive evaluation of cardiac function have contributed to significant advances in the diagnosis of diastolic HF. The development of specific criteria for the diagnosis of diastolic HF should incorporate modern non-invasive techniques for assessment of diastolic function within a clear framework of clinical evaluation, as suggested in fig 13. Such an approach should form the cornerstone for entry into urgently needed trials of therapeutic intervention for this important condition.
Stepwise approach to clinical evaluation of the dyspnoeic patient with normal LV systolic function for the presence of diastolic heart failure.
Assessment of diastolic function: key points
-
A large proportion of patients presenting with heart failure have preserved left ventricular (LV) systolic function, but evidence of abnormal diastolic function
-
Assessment of diastolic function is complex, and involves the interpretation of multiple load dependent parameters
-
An “impaired relaxation” pattern of LV filling needs to be interpreted in the context of other echo and clinical variables, and may be clarified by the use of exercise testing
-
Differentiating between normal and pseudonormal LV filling patterns is clinically important and can be performed with careful interpretation of the transmitral and pulmonary venous Doppler flows in combination with tissue Doppler velocities, LV flow propagation velocity, and dynamic manoeuvres.
-
In patients with preserved systolic function, measurement of transmitral flow in combination with mitral annular tissue Doppler velocities provide the most efficient means of assessing LV diastolic function and filling pressures
-
In patients with systolic dysfunction, diastolic Doppler parameters are principally used to determine LV filling pressures
-
Paroxysmal or permanent atrial fibrillation limits the interpretation of diastolic Doppler parameters
-
BNP correlates well with LV filling pressures in symptomatic patients with low ejection fraction, but is less useful in patients with preserved LV systolic function
-
New non-invasive imaging techniques may allow early detection of myocardial dysfunction
Additional references appear on the Heart website—http://www.heartjnl.com/supplemental
Acknowledgments
We wish to thank Dr Roess Pascoe for assistance in obtaining some of the echo images.
REFERENCES
Supplementary materials
The references are available as a downloadable PDF (printer friendly file).
If you do not have Adobe Reader installed on your computer,
you can download this free-of-charge, please Click hereFiles in this Data Supplement:
- [view PDF] - Assessment of diastolic function: what the general cardiologist needs to know - web only references
Footnotes
-
Take the online multiple choice questions associated with this article (see page 695)
Linked Articles
- Miscellanea
- Miscellanea
- Miscellanea
- Miscellanea